Once the reference Member State Competent Authority (MSCA) has validated the application for the renewal of the national authorisation of a biocidal product (or a biocidal product family) granted by mutual recognition, the evaluation process starts.
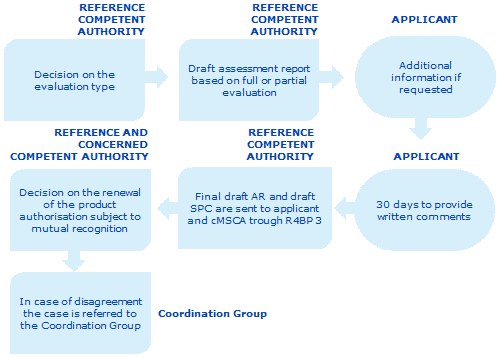
This graphic shows an overview of the dossier evaluation process
Steps
The process for the renewal of national authorisation subject to or granted through mutual recognition has the following steps:
The reference MSCA has 90 days from the validation date to assess whether a full evaluation of the dossier is required.
The reference MSCA drafts an assessment report and the conclusions of its evaluation within 365 days of validating the application, where a full evaluation is required. The assessment report includes the reasons for deciding whether or not to renew, the authorisation. If a full evaluation is not needed, the reference MSCA has to decide on the renewal of the authorisation within 180 days of accepting the application.
If the reference MSCA considers that additional information is necessary to carry out a full evaluation, it can request this from the applicant. The applicant has to provide the requested information within 180 days unless a delay is justified by the nature of the data requested or by exceptional circumstances.
The draft assessment report is sent through R4BP 3 to the applicant who given the opportunity to submit comments within 30 days. The reference MSCA must take account of any comments from the applicant when finalising the assessment report.

The final draft assessment report and, if the draft assessment report is positive the draft summary of product characteristics, are sent to the applicant and to the concerned MSCAs through R4BP 3.

The concerned MSCAs express their position on the draft summary of product characteristics within 90 days. In case of agreement (and a positive assessment report), the reference and concerned MSCAs renew the national authorisations within 30 days of reaching agreement.

If a concerned MSCA does not agree on the conclusion of the assessment report or on the summary of product characteristics, the case will be referred to the Coordination Group by the concerned MSCA.
Actors
The main actors in the evaluation process are:
Applicants
Applicants are responsible for providing all necessary information in their dossiers. They should pay attention to the various deadlines throughout the process. Applicants can comment on the draft assessment report on their dossier during the process.
Reference MSCA
The reference MSCA is responsible for validating the application dossiers and for evaluating the applicant's dossier in case of a mutual recognition renewal. It can be a different MSCA then the one that evaluated the original authorisation. In case of disagreement with the concerned MSCA on the summary of the biocidal product characteristics it refers the matter to the Coordination Group.
Concerned MSCA
The concerned MSCAs agree on the summary of the biocidal product characteristics issued by the reference MSCA and renew the authorisation in case of agreement. In case of disagreement the concerned competent authority gives a detailed statement to the other concerned MSCAs and to the applicant on the reasons for its position and the case is referred to the Coordination Group (CG).
Coordination Group
The coordination group CG is a body formed by representatives of the MSCA and the Commission to solve disagreements in relation to mutual recognition and simplified notification procedures. ECHA provides the secretariat for the CG