Q&As
Q&As
Q&A info
Trid tfittex il-mistoqsija u t-tweġiba rilevanti fil-lingwa tiegħek? Ibdel il-lingwa fil-menù li jinżel hawn fuq.
Back
The obligations of the importer of the mixture depend on the interpretation of each specific entry in Annex XVII to REACH for the substance concerned, taking account of the wording, the context and the purpose of the restriction in question.
For instance, if a substance were completely banned, then it could not be placed on the market, not even as an impurity in a substance in an imported mixture. On the other hand, some Annex XVII entries specify limits above which a substance cannot be placed on the market. This limit may not be exceeded, no matter what is the source of the substance in the mixture. However, this can only be determined on a case-by-case basis depending on the substance, the restriction and the concentration of the substance as an impurity in the imported mixture.
It should be noted that the impurity may be permissible at any concentration if the use of the imported mixture is not covered in the ‘conditions of restriction' listed in Annex XVII for the substance.
In special cases where, due to the size or shape of the packaging, it is technically not possible to include the protective gloves inside the packaging, it is considered to be sufficient that the gloves are fixed tightly to the packaging in a manner that they cannot be unintentionally removed during handling and transport. The gloves must not obstruct the label and the removal of the gloves must not destroy the label. In addition, both the packaging containing the mixture and the protective gloves must be placed on the market as a single unit, which explicitly signals to the consumer that the mixture may only be used with the protective gloves .
The new legal act may explicitly state that references to the repealed act must be construed as references to the new legal act (e.g., Article 139 of REACH).
For example:
- Entry 19 (paragraph 4) exempts certain uses of arsenic compounds for wood preservation if they are authorised in accordance with Directive 98/8/EC. This Directive was replaced by Regulation (EU) 528/2012, which explicitly states that references to the repealed Directive must be interpreted as reference to the new regulation (Article 96).
- Entry 45 (paragraph 3) exempts electrical and electronic equipment within the scope of Directive 2002/95/EC from the restriction of diphenylether, octabromo, derivative. This Directive was replaced by Directive 2011/65/EU, which explicitly states that references to the repealed Directive must be interpreted as reference to the new directive (Article 26).
- Entry 50 (paragraph 3) defines tyres covered by the PAH restriction as tyres for vehicles covered by three directives, including Directive 2002/24/EC. This Directive was replaced by Regulation (EU) 168/2013, which expressly states that references to the repealed Directive must be interpreted as reference to the new regulation (Article 81).
Article 31(6) of the REACH Regulation provides that the safety data sheet (SDS) shall contain a Section 15 entitled ‘regulatory information’. Annex II to REACH provides requirements for the compilation of the SDS. Section 15(1) specifically mentions that, if the substance or mixture covered by the SDS is the subject of specific provisions in relation to the protection of human health or the environment at Union level (e.g. restrictions under Title VIII), these provisions must be mentioned, unless this information is already mentioned in other parts of the SDS. Thus, all restriction entries applicable to the specific substance or mixture covered by the SDS need to be indicated therein. As an example, SDSs including carcinogenic, mutagenic or toxic to reproduction substances (as such or in a mixture) listed in appendices 1 to 6 to REACH, need to refer to entries 28, 29 or 30 of Annex XVII. If another specific restriction exists for these substances, this needs to be mentioned as well in the SDS.
Moreover, Article 31(9) of the REACH Regulation requires suppliers to update the SDS without delay once a restriction has been imposed. In Section 16 (other information), a clear indication of where changes to the previous version have been made needs to be included, unless such indication is given elsewhere in the safety data sheet, with an explanation of the changes.
To further clarify the exemption (within Article 67(1) of the REACH Regulation, manufacture, placing on the market or use of a substance in scientific research and development (SRD) is exempted for restrictions), note that under the authorisation process the following Q&As (concerning the exemption in Article 56 for the use of Annex XIV substances in scientific research and development) has been provided. The same approach can be broadly considered as applicable to restrictions.
- Q&A 1153 states that sampling for further analysis is not exempted and thus not regarded as scientific research and development. However, “activities considered to form part of the use of the sample in performing analytical activities” fall within the exemption.
- Q&A 1030 explains that the uses of a substance upstream preceding an exempted end-use in scientific research and development are also exempted in quantities of the substance ending up in SRD (i.e. under 1 t/y per user) subject to certain conditions.
- Q&A 585 explains that the exemption from authorisation also applies to the use of a substance in analytical activities such as monitoring and quality control under certain conditions. This exemption applies irrespective of where the analysis is performed i.e. on-site or off-site facilities, but does not cover sampling activities.
Many entries in the Restriction List (Annex XVII) cover articles. Such entries are, for instance, entries 50 - 52, 61 and 63. These may address types of textiles and leather articles, even if these are not explicitly mentioned.
The following entries in the Restriction List (Annex XVII) are specific to textiles:
- Entry 4 ((2,3 dibromopropyl) phosphate, CAS No 126-72-7);
- Entry 7 (Tris(aziridinyl)phosphinoxide, CAS No 545-55-1; EC No 208-892-5) and
- Entry 8 (Polybromobiphenyls; Polybrominated biphenyls (PBB), CAS No 59536-65-1).
These entries state that these substances “Shall not be used in textile articles, such as garments, undergarments and linen, intended to come into contact with the skin.”
The following entries in the Restriction List (Annex XVII) restrict substances in relation to textiles and/or leather articles:
- Entry 18, restriction on mercury compounds in the impregnation of heavy-duty industrial textiles and yarn intended for their manufacture;
- Entry 20 (paragraph 6), restriction on dioctyltin (DOT) compounds in textile articles intended to come into contact with the skin;
- Entry 23 (paragraph 6), restriction on cadmium and its compounds in textiles and clothing;
- Entry 43, restriction on azocolourants and azodyes in textile and leather articles which may come into direct and prolonged contact with the human skin or oral cavity (indicative list is provided);
- Entry 46 (paragraph 3) restriction on nonylphenol and nonylphenol ethoxylates in textiles and leather processing (with some exceptions);
- Entry 46a, restriction on nonylphenol ethoxylates in textile articles which can reasonably be expected to be washed in water during their normal lifecycle, and
- Entry 47 (paragraphs 5-7), restriction on chromium VI compounds in leather articles coming into contact with the skin; and
- Entry 72 (paragraph 1), restriction on specific substances listed in column 1 of the Table in Appendix 12 in i) clothing and related accessories, ii) textiles other than clothing which, under normal or reasonably foreseeable conditions of use, come into contact with human skin to an extent similar to clothing and iii) footwear if they are for use by consumers (with some exceptions).
The following entries of the Restriction List (Annex XVII) specifically include a derogation for electrical and electronic equipment:
Entry 45 (paragraph 3) provides a derogation from the restriction on diphenylether, octabromo derivative (C12H2Br8O) for electrical and electronic equipment within the scope of Directive 2002/95/EC, which has been replaced by Directive 2011/65/EU (on the restriction of the use of certain hazardous substances in electrical and electronic equipment), and
Entry 63 (paragraph 8) excludes articles within the scope of Directive 2011/65/EU from the restriction on lead and its compounds in articles supplied to the general public.
In addition, the following entries in the Restriction List (Annex XVII) explicitly restrict the placing on the market of paints and/or paint strippers:
Entry 16, restriction on certain lead carbonates in substances or mixtures intended for use as paint;
Entry 17, restriction on certain lead sulphates in substances or mixtures intended for use as paint;
Entry 20, restriction of organostannic compounds acting as biocide in free association paint;
Entry 20 (paragraph 5), restriction on dibutyltin (DBT) compounds in paints and coatings;
Entry 23 (paragraph 2), restriction on cadmium and its compounds in paints with codes [3208] and [3209] and in painted articles;
Entries 28-30 (paragraph 1), restriction on CMRs as substances, as constituents of other substances or in mixtures (including paints), for supply to the general public; (paragraph 2) derogation for artists’ paints covered by Regulation (EC) No 1272/2008;
Entry 48, restriction on toluene (CAS No 108-88-3; EC No 203-625-9) in spray paints intended for supply to the general public;
Entry 54, restriction on (2-(2-methoxyethoxy)ethanol (DEGME) (CAS No 111-77-3; EC No 203-906-6) for supply to the general public, as a constituent of paints and paint strippers;
Entry 55, restriction on 2-(2-butoxyethoxy)ethanol (DEGBE) (CAS No 112-34-5; EC No 203-961-6) for supply to the general public, as a constituent of paints and paint strippers and in spray paints and
Entry 59, restriction on dichloromethane in paint strippers under certain conditions.
In general, cleaning refers to any removal of dirt or pollution from articles or places, both in industrial and institutional facilities as well as in households.
In some entries cleaning may be specified by reference to a particular user group(s) or potential exposure pattern to which the restriction applies. For example, column 2, paragraph 1 of entries 32-38 refer to the substance or mixture being intended for supply to the general public and/or intended for diffusive applications where releases can occur from multiple sources such as surface cleaning and cleaning of fabrics. Column 2, paragraph 1 of entry 46 applies to industrial and institutional cleaning systems (e.g. in schools, hospitals), with the exception of dry cleaning done in a controlled closed system where the washing liquid is recycled or incinerated and other cleaning systems where special treatment is used with recycling or incineration of the washing liquid. Column 2, paragraph 2 entry 46 applies to all domestic cleaning.
The term ‘article’, as interpreted by the European Court of Justice (ECJ) in its judgement of 10 September 2015 in case C-106/14 applies in the same way to restrictions in Annex XVII as to the other aspects of the REACH Regulation. The judgement is available here: http://curia.europa.eu/juris/liste.jsf?language=en&td=ALL&num=C-106/14.
For the purposes of REACH, the term 'article' has the specific meaning set out in Article 3(3) of the REACH Regulation. Article 3(3) defines an article as ‘an object which during production is given a special shape, surface or design which determines its function to a greater degree than does its chemical composition’.
'Complex objects' are made up of more than one article which meet the criteria laid down in Article 3(3) of REACH, e.g. a bicycle is a complex object made up of several articles, such as handlebar grips, cables, screws etc. Complex objects are explained in the ECHA Guidance on requirements for substances in articles. Please note the term ‘complex object’ corresponds to ‘complex product’ that is used in the ECJ Judgement referred to above (see footnote 12 of the above guidance).
The ECJ, in its judgment, observed that the REACH Regulation does not contain any provisions specifically governing complex products and that consequently, in the absence of specific provisions, there is no need to draw a distinction between articles of their own (e.g. screw) or when incorporated as components of a complex product (e.g. a screw in a bike). Therefore, when incorporated into a complex product, an 'article' remains an article within the meaning of REACH, as long as the article retains its special shape, surface or design, which is more decisive for its function than its chemical composition.
Entries in Annex XVII restricting 'articles' cover any object meeting the criteria in Article 3(3) of the REACH Regulation. Therefore, if an entry restricts the placing on the market of articles containing/releasing substance X and the restriction affects complex objects, the presence/release of substance X in each of the individual articles should be checked.
If a restriction entry refers to ‘parts of articles’, this should be understood as referring to an integral part of an article. Please note questions and answers (Q&As) and guidelines for some entries in Annex XVII have been developed which provide further information. The Q&As and guidelines are available on the ECHA website here: https://echa.europa.eu/information-restricted-substances.
A number of Annex XVII entries (Entries 3, 20, 28-30, 32-38, 40, 48, 50, 54, 55, 56, 57, 59, 63(7) and 73) specifically refer to the ‘supply to the general public’ in their conditions to the restriction. Substances, mixtures or articles are considered to be for supply/supplied to the general public if they are to be placed on the market for end-users belonging to that group (e.g. final consumers).
The fact that a substance, mixture or article is supplied between economic operators before arriving to the general public, does not exempt it from any of the conditions (e.g. concentration limits) imposed by Annex XVII entries that make reference to ‘for supply to the general public’. Also substances, mixtures or articles put at the disposal of the general public in a public area fall within the definition of ‘supply to the general public’, irrespective of the type of ownership (public or private) or transaction by which they were put at their disposal. As an example, in the restriction entry 50, paragraph 5, it is understood that tiles/mats used in public playgrounds are supplied to the general public when they are put at the disposal of the general public.
It is to be noted that some restriction entries have general restriction ‘placing on the market’ without referring to any specific group, thus covering also supply to the general public (e.g. entry 63(1)). Entry 69 refers to ‘placed on the market to the general public’, which is considered to have similar meaning than ‘supply to the general public’.
Substances identified as Persistent Organic Pollutants (POPs) are regulated worldwide by the Stockholm Convention and the Aarhus Protocol. These international agreements are implemented in the EU by Regulation (EU) No 2019/2021 (POPs Regulation).
When a POP substance is included in the Annexes I, II, III or IV to the Regulation (EU) 2019/2021, the POPs Regulation applies. The manufacturing, placing on the market and use of substances listed in Annex I or II of POPs is prohibited or severely restricted (some exemptions apply).
When a substance already restricted under REACH is included in Annex I or II to POPs, the Commission removes the corresponding entry from Annex XVII of REACH by a separate amendment to REACH. For example, substances that were included in restriction entries 22, 42, 44, 53 and 67 of Annex XVII to REACH have been identified as POPs and these entries have been deleted. PFOA substances included in restriction entry 68 have also been identified as POPs and restriction entry 68 has been amended by removing PFOAs and including C9-14 PFCAs.
You can find information on the deleted entries and the related Commission Regulations under ‘Further information on the table’ on the ECHA website in the Substances restricted under REACH section.
Substances included into an Annex to the POPs Regulation should be removed from Annex XVII of REACH. However, it may happen that the inclusion into the POPs list and deletion from Annex XVII do not occur simultaneously. In such a case, the most restrictive conditions (usually under the POPs Regulation) apply.
Restriction entry 68 was first included into Annex XVII to REACH by Commission Regulation (EU) 2017/1000 on 14 June 2017 and it initially covered Perfluorooctanoic acid (PFOA), its salts and PFOA-related substances. PFOA substances have been further included in the list of substances restricted under the POPs Regulation (Regulation (EU) 2019/1021) in June 2020 and consequently removed from Annex XVII of REACH. A new entry 68 of Annex XVII to REACH (same entry number but new substances) was subsequently included by Commission Regulation (EU) 2021/1297 of 4 August 2021, as corrected, which restricts the placing on the market and use of perfluorocarboxylic acids containing 9 to 14 carbon atoms in the chain (C9-C14 PFCAs), their salts and C9-C14 PFCA-related substances.
A plastic article is an article made entirely of plastic materials or an article containing components constituted by plastic materials. REACH Regulation doesn’t define plastic, however a definition of plastic is available in Article 3(1) of Directive (EU) 2019/904 as: ‘a material consisting of a polymer as defined in point 5 of Article 3 of Regulation (EC) No 1907/2006, to which additives or other substances may have been added, and which can function as a main structural component of final products, with the exception of natural polymers that have not been chemically modified’.
In the REACH regulation, a number of restriction entries apply directly to articles made of plastic materials. There are other entries that apply to articles in general, that include also articles made of plastics. However, the possible presence of certain restricted substances in plastic articles has to be assessed on a case-by-case basis.
Entries related mostly to plastic articles:

Entries related to specific categories of articles:

Restriction Entries covering all articles:

ECHA has developed a ‘Compendium of Analytical Methods’ to provide a ready reference of some available analytical methods that authorities or industry may use in order to assess the compliance of chemicals manufactured, used or placed on the Union market with the restrictions set forth in Annex XVII to REACH. If a test method is specified in the legal text, it has to be adhered to.
For instance, for Entry 27 (Nickel and its compounds) there are 3 available analytical methods recommended by the ECHA Forum that can be used in the verification of compliance of this entry:
As of April 2021, the titles and references of standards to be used as the analytical methods for demonstrating the conformity of articles under entry 27 of Annex XVII to REACH will be published on the Commission website only and no publication in the Official Journal of the European Union is foreseen anymore. Furthermore, updated references and titles of standards relevant for entry 27 can be found in both the restrictions webpage and the harmonised standards webpage of DG GROW.
Concerning the Restriction on CMRs 1A and 1B in Textiles and Clothing, a list of available analytical is available in the document Explanatory Guide on the Restriction on CMRs 1A and 1B in Textiles and Clothing.
Lastly, information on test methods is available in each of the background documents accessible in the Registry of restriction intentions.
A specific frequency for the update of Annex XVII is not indicated in the REACH regulation. The List of restricted substances (Annex XVII, REACH) is updated by amendments of the REACH Regulation. These amendments may result in the introduction of a new restriction entry, modifications or deletion of an existing entry.
The timelines for amending Annex XVII of REACH are in the remit of the European Commission. In addition, Annex XVII contains certain descriptive entries covering substances listed in appendices, which are also updated when new substances fall under the respective entries (e.g., when new harmonised classifications are laid down for substances and which have been included in the appendices by the Commission Regulation). It is possible to follow the changes of a given entry throughout the years under ‘History’ in the entry-specific web page on the ECHA’s website in the Substances restricted under REACH section.
In addition, ECHA's website includes a Registry of restrictions intentions in order to allow interested parties to be aware of the substances for which the authorities intend to submit Annex XV dossiers for restrictions.
Article 67(1) of the REACH Regulation states that a substance in its on, in a mixture or in an article, for which Annex XVII contains a restriction, cannot be manufactured, placed on the market or used unless it complies with the conditions of that restriction.
On the basis of Article 69(1) of REACH the Commission may request, ECHA, to start a restriction procedure when they are concerned that the manufacture, placing on the market or use of a certain substance poses a risk to human health or the environment that is not adequately controlled and needs to be addressed. A Member State (or a group of Member States) can also start a restriction procedure on the basis of Article 69(4) of REACH.
On the Basis of Article 69(2), when the sunset date of a substance included into Annex XIV or REACH (Authorisation List) has passed, ECHA verifies if the use of that substance in articles may pose a risk to human health or the environment. If ECHA concludes that the risk is not adequately controlled, they have to submit a restriction proposal (Annex XV dossier) to the Commission.
The restriction procedure applicable to these cases is regulated in Articles 69-73 of REACH and explained on the ECHA website in the Restriction procedure page.
Note that to communicate about the upcoming proposal in advance, the intention to prepare a restriction proposal is made public in the registry of intentions before the proposal file itself is prepared.
The phases (and their duration) of this restriction process are:
1) Preparation and submission of a restriction proposal (12 months) – Phase 1
2) Public consultations on the restriction report and the SEAC’s draft opinion (6 months) – Phase 2a
3) Opinion development of ECHA’s Committee for Risk Assessment (RAC) and Committee for Socio-economic Analysis (SEAC) (12 months since publication of the proposal) – Phase 3.1
4) If the Commission considers that the conditions for a restriction are fulfilled, it prepares a draft amendment to the list of restrictions in Annex XVII to REACH (within 3 months of receipt of the SEAC opinion). Commission decision in a comitology procedure (duration varies). And finally, complying with and enforcing the restriction – Phase 3
The Commission can also propose restrictions for consumer use as regards substances on their own, in mixtures or in articles, which meet the criteria for classification as carcinogenic, germ mutagenic or toxic for reproduction category 1A or 1B and could be used by consumers (Art. 68(2) REACH). This process follows a simplified path which does not require consultations by RAC and SEAC.
Interested parties can follow all phases of a restriction proposal from the notification of the intention, to the adoption of the final opinions by the Committee for Risk Assessment (RAC) and the Committee for Socio-economic Analysis (SEAC), till the adoption of the restriction by the European Commission by amending Annex XVII of REACH.
Stakeholders are encouraged to submit any relevant information to the dossier submitters during the preparation of the restriction proposal by responding to specific Call for Evidence published in ECHA’s website or in the website of the Member State Authority who is preparing the proposal.
Once the proposal is submitted, information can be provided to support the decision-making process by responding to the public consultations on the restriction proposal (once it is submitted) and on SEAC draft opinion. Advice on the public consultation and dedicated procedure on how to submit information are provided in the ECHA’s website.
More information on the restriction process can be found on the ECHA website.
This entry refers to hazard classes set out in Regulation (EC) No 1272/2008 on the classification, labelling and packaging of substances and mixtures (CLP Regulation). A full list of the hazard classes referred to in this entry with their corresponding hazard categories and hazard statements is provided in this table.
The REACH Regulation does not provide a specific definition for paints.
According to a general meaning, paint is a mixture, usually of a liquid with a solid pigment. Furthermore, Commission Decision 2009/544/EC (establishing the ecological criteria for the EU Ecolabel to indoor paints and varnishes) provides the following definition: 'Paint' means a pigmented coating material, in liquid or in paste or powder form, which when applied to a substrate, forms an opaque film having protective, decorative or specific technical properties.
The abovementioned definitions provide an indication of what could be generally considered as a paint in the context of entries 16-17 of Annex XVII (to REACH) provisions.
It should be noted that the restrictions in entries 16-17 of Annex XVII only allow Member States to permit the use of paints containing the restricted substances for the restoration and maintenance of works of art and historic buildings and their interiors. Thus children's paint sets and other stationery-type paints such as artist paints and do-it-yourself (DIY) decorations for t-shirts must not contain the restricted substances.
Concerning children's paint sets, these may also be covered by the Toys Directive (Directive 2009/48/EC on the safety of Toys), which lays down limits for the presence of lead in toys.
The REACH Regulation does not provide a definition of measuring devices (often referred to as measuring instruments). However, in the context of entry 18(a), it has to be noted that:
- The restriction provision in paragraph 1 of entry 18(a) covers those measuring devices that are intended for sale to the general public and provides an indicative list of these (thermometers, manometers, barometers, sphygmomanometers etc.)
- Furthermore paragraphs 5 and 7 provide exhaustive lists of mercury-containing measuring devices intended for industrial and professional uses which have not been allowed to be placed on the market after 10 April 2014 (barometers; hygrometers; manometers; sphygmomanometers; strain gauges to be used with plethysmographs; tensiometers; thermometers and other non-electrical thermometric application; mercury pycnometers; mercury metering devices for determination of the softening point; mercury triple point cells other than those used for the calibration of platinum resistance thermometers).
For the purposes of Directive 2004/22/EC on measuring instruments, ‘measuring instrument' means any device or system with a measurement function that is covered by Articles 1 and 3. According to the European Standardisation organisation-CEN, "measuring instruments allow for testing the accuracy and calibration of measuring devices (e.g. water meters, gas meters, electricity meters, etc.)". (http://www.cencenelec.eu/standards/sectors/mid/pages/default.aspx)
The abovementioned definitions provide an indication of what could generally be considered as a measuring device in the context of entry 18(a) of Annex XVII to REACH provisions.
Paragraph 4b) of Annex XVII to REACH concerning arsenic compounds provides for a list of applications for which wood treated with CCA may be used. This is not a list of examples of possible uses but an exhaustive list of authorised applications. It flows both from the actual wording of those provisions and from their objective that the derogation provided for in paragraph 4 must necessarily be subject to a strict interpretation, as confirmed by the Court of Justice (case C-358/11, pp. 40-43).
Consequently, wood treated with CCA cannot be used for other applications than the ones listed in paragraph 4 b). Wood treated with CCA can, therefore, not be used for railway sleepers installed above ground.
Organostannic compounds covered by entry 20 in Annex XVII to REACH, must contain a carbon-tin bond. Substances like tin salts or organotin compounds, for which tin is bound to an atom other than carbon (for example hexanoic acid, 2-ethyl-, tin(2+) salt (CAS-No. 301-10-0)) are not covered by entry 20 in Annex XVII to REACH.
(a) Yes, they do. Entry 20 in the Restriction List (Annex XVII) to REACH imposes DOT compounds restrictions for childcare articles, which are not toys. The REACH Regulation does not contain a definition of toys. The definition of toys in Directive 2009/48/EC on the Safety of Toys is illustrative in determining what should be considered as a “toy” in the context of this restriction (see Q&A 0982).
However, paragraph 6(a) of entry 20 restricts DOT compounds in textile toys as in any other textile article intended to come into contact with the skin. Furthermore, organic tin (including DOT) in toys is restricted by paragraph 13 of Part III (Chemical Properties) of Annex II to Directive 2009/48/EC on the safety of toys, which specifies maximum migration limits.
(b) and (c)
In addition to derogation in paragraph 5(d), the other derogations currently applicable to TBT, DBT and DOT compounds in articles relate to the continued placing on the market of articles that were already in use in the EU before the 1 July 2010 for TBT (paragraph 4(b), before the 1 January 2012 (paragraph 5(b)) for DBT and before the 1 January 2012 for DOT (paragraph 6(b)).
Yes. Packaging can be considered as an article (or a product composed of different articles) in its own right. More information can be found in the ECHA guidance on requirements for substances in articles available at https://echa.europa.eu/guidance-documents/guidance-on-reach (in particular in chapter 2).
Therefore, with regard to entry 20, packaging should comply with the restrictions for tri-substituted (TBT, TPT) and dibutyltin (DBT) compounds. The restriction of dioctyltin compounds (DOT) which applies only to certain listed articles for the general public, applies to textile packaging.
Compliance with the restriction is assessed based on the weight of the entire painted article and not based on the weight of the layer of dry paint. This approach follows the scenarios from the Guidance on requirements for substances in articles, Table 5 under subchapter 3.2.3.1.
For further details see Q&A 1564 on Annex XVII and concepts of ‘article’, ‘complex object’ and ‘parts of articles’.
With reference to paragraph 10 of the Annex to Commission Regulation (EU) 494/2011 amending entry 23 of Annex XVII of the REACH Regulation (cadmium) the concentration threshold of cadmium applies in each metal part of jewellery. The wording used by the legislator, i.e. "metal parts of the jewellery and imitation jewellery" implies that each metal part is relevant; therefore in order to determine if the restriction applies the calculation of the concentration in this case is to be done for each metal part. Therefore, if there are several metal layers as coatings on the surface of an inner (metallic) part of the jewellery these should be regarded as integral part of the metal part and the concentration limit of 0,01% is calculated for this whole metal part. In case the inner part is not metal, but the coating is made of metal layers, this coating is regarded as one metal part. If the jewellery article contains several metal parts, each of them should comply with the concentration limit.
The prohibition of the placing on the market of jewellery and imitation jewellery articles containing cadmium includes sales from the manufacturers to distributors and from distributors to retailers, as well as imports. However, Commission Regulation (EU) 494/2011 contains derogation for articles that were placed on the market before 10 December 2011 (for the date see corrigendum published in OJ L 136/105). This means that jewellery and imitation jewellery articles placed on the market for the first time before 10 December 2011 do not need to comply with the prohibition thus they can be sold following entry into force of the new restriction for example to a retailer or on the second-hand market.
Paragraph 8 of entry 23 of Annex XVII to the REACH Regulation states that cadmium and its compounds shall not be used in brazing fillers in a concentration equal to or greater than 0,01 % by weight. In addition, brazing fillers shall not be placed on the market if the concentration of cadmium (expressed as Cd metal) is equal to or greater than 0,01 % by weight. The paragraph also states that brazing shall mean a joining technique using alloys and undertaken at a temperature above 450°C. In accordance with the following paragraph 9, by way of derogation paragraph 8 shall not apply to brazing fillers used in defence and aerospace applications nor to brazing fillers used for safety reasons.
The safety aspect in relation to this derogation is if the use of cadmium containing brazing filler may prevent accidents causing human suffering or environmental pollution.
For the enforcement purposes examples of applications are given. It can be considered that the derogation on uses of cadmium containing brazing fillers for safety reasons in paragraph 9 of entry 23 covers the current uses, such as:
-
Brazing fillers used in turbine wheels in power plant technology in temperature below 650°C.
Turbine wheels in power plant technology are parts of speed drivers for gas compressors and boiler feed pumps, where rotational speed is approximately from 1000 revolutions per minute (rpm) up to 20 000 rpm. Cadmium containing brazing fillers are needed as they can be used below 650 °C without decreasing the strength of the parent material (base metal). Cadmium-free brazing fillers require higher temperatures which causes the weakening of the parent material. Weakening of the parent material could lead to the breakdown of the turbine wheel. Due to high rotational speed the parts and pieces of shrapnel may cause injuries to workers and others in the vicinity of the wheel. The breakdown of the turbine wheel may result also in a complete shutdown of the power plant, the compressor station of a gas pipeline, or of a refinery.
-
Brazing fillers used in pipes and tubes where acetylene is transferred in high pressure (1.5 – 17 bar).
Cadmium containing brazing fillers are needed in the joining process for pipes and tubes, where acetylene is transferred in order to avoid formation of explosive substances. Acetylene forms explosive substances with copper and silver as well as other materials (e.g. formation of copper acetylide and silver acetylide). Cadmium reduces the overall percentage of copper and silver in the brazing fillers to the level where formation of explosive substances does not exist. Another reason to use cadmium in brazing fillers for this application is that cadmium facilitates the capillary action and solder penetration ensuring a good quality joint with high integrity for pipes and tubes where acetylene is transferred in high pressure (1.5-17 bar). Release of acetylene from pipes and tubes may as well cause serious risk, as acetylene is extremely flammable gas and explosive with and without contact with air.
Other applications that would like to benefit from the derogation need to show the similar kind of safety aspects as described above.
Such considerations should take into account the availability on the market of cadmium-free brazing fillers which can address the safety aspects of the specific application of the brazing fillers in an equivalent manner.
See also ECHA´s report "The use of brazing fillers containing cadmium for safety reasons" [PDF].
Paragraph 1 of entry 23 of Annex XVII to the REACH Regulation provides a restriction on cadmium and its compounds in mixtures and articles produced from certain synthetic organic materials (plastic materials) and paragraph 2 provides a restriction on cadmium and its compounds in paints (Tariff codes 3208 and 3209). The following paragraph 3 states that by way of derogation the restrictions in paragraphs 1 and 2 do not apply to articles coloured with mixtures containing cadmium for safety reasons.
There are two safety aspects in relation to this derogation. The first relates to the use of a specific colour or pigment with certain properties which is necessary to prevent accidents. The second relates to the use of a specific colour or pigment with certain properties in safety equipment.
For the enforcement purposes example of applications are given. Based on above, it can be considered that the derogation in paragraph 3 of entry 23 covers current applications of articles such as:
-
Coloured wire insulation and cable jackets used in aircraft electrical and control systems for the purpose of fire detection and extinguishing systems, flight control systems or during flight tests.
The wire and cable connections are often used in in a high temperature application (greater than 150ºC ambient temperature). Cadmium pigments are used to keep the colour from changing or fading over time in the high temperature. Changing established colour conventions could introduce a significant risk of maintenance errors, which may lead to a risk of passengers.
-
Outdoor safety equipment, such as:
- parts of rescue boats for ships (e.g. safety belts, water pockets of life rafts, canopies) and
- parts of safety equipment for outdoor applications (e.g. seats, reels and diverse technical parts).
The other applications that would like to benefit from the derogation need to show the similar kind of safety aspects than described above.
Such considerations should take into account the availability on the market of alternative substances which can address the safety aspects of the specific application in an equivalent manner.
See also ECHA´s report "The use of cadmium and its compounds in articles coloured for safety reasons" [PDF].
Entry 23 (paragraph 10 (i)) of Annex XVII to the REACH Regulation states that cadmium and its compounds must not be used or placed on the market if the concentration of cadmium is equal to or greater than 0,01 % by weight of the metal in metal beads and other metal components for jewellery making (see Q&A, 158). In addition, it should be noted that articles produced from plastic material referred to in paragraph 1 of the entry must not be placed on the market if the concentration of cadmium is equal to or greater than 0.01 % by weight of the plastic material.
Thus, in the case of plastic coated metal beads (CCB beads), both the plastic material and the metallic part of the bead need to comply with entry 23 (cadmium restriction).
Please also note that if the article is painted, then paragraph 2 will also apply to this article.
Note that metallic articles containing cadmium may be covered under other EU legislation. For example, (1) electrical and electronic equipment falls under Directive 2011/65/EU on the Restriction of the use of certain Hazardous Substances and must comply as well with the maximum concentration limits for cadmium set in that Directive and (2) toys are covered under Directive 2009/48/EC on the Safety of Toys.
Entry 27 of Annex XVII to REACH states that nickel may not be used "in articles intended to come into direct and prolonged contact with the skin, if the rate of nickel release from the parts of these articles coming into direct and prolonged contact with the skin is greater than 0.5 Jg/cm²/week". The aim of this restriction to protect consumers against nickel allergy which may be caused by prolonged contact of the skin with nickel-releasing articles that come into direct and prolonged contact with the skin such as jewellery, buttons, tighteners, zips and rivets in items of clothing. It has emerged that some mobile telephones contain nickel in surface material and that consumers are at risk of developing eczema through skin contact with the mobile telephone. As mobile telephones are clearly intended to come into direct contact with the skin, and as they are used on a daily basis often for prolonged periods of time, it is considered that mobile telephones fulfil the condition of "direct and prolonged contact with the skin". Therefore mobile telephones are covered by the restriction and should comply with the conditions set in Entry 27 of Annex XVII to REACH.
Prolonged contact with the skin is defined as contact with the skin to articles containing nickel of potentially more than
- 10 minutes on three or more occasions within two weeks, or
- 30 minutes on one or more occasions within two weeks.
The skin contact time of 10 minutes applies when there are three or more occasions of skin contacts within a two-week time period. The skin contact time of 30 minutes applies when there is at least one occasion within a two-week time period.
This entry covers:
- post assemblies that are inserted into pierced parts of the human body and release nickel at a rate equal or greater than 0,2 μg/cm2/week;
- any article intended to come into direct and prolonged contact with the skin that releases nickel at a rate greater than 0,5 μg/cm2/week from its parts coming into direct and prolonged contact with the skin. The restriction applies regardless of the materials from which the articles are produced.
The term ‘Prolonged contact with the skin’ is clarified in the Q&A 935.
Substances within the scope of entries 28-30 are not allowed to be placed on the market or used for supply to the general public as substances, as constituents of other substances or in mixtures when the concentration is equal to or greater than the specified limits. Certain derogations apply to this restriction as listed in paragraph 2. CMR substances that are present in articles are not within the scope of the restriction imposed by entries 28-30, but other restrictions may be applicable to these substances, when present in articles. In addition, notification and communication obligations under REACH may apply; see the ECHA website: https://echa.europa.eu/regulations/reach/candidate-list-substances-in-articles/notification-of-substances-in-articles.
It should be noted that entries 28 and 29 are applicable to the substances that are listed in Appendices 1 & 2 (carcinogenic (C), categories 1A and 1B) and Appendices 3 & 4 (mutagenic (M), categories 1A and 1B). Entry 30 is applicable to substances which are classified as reproductive toxicants (R), categories 1A and 1B and listed in Appendices 5 and 6. These Appendices are regularly updated by including new substances, after the adoption of a harmonised classification for a substance as CMR, category 1A or 1B according to Regulation (EC) No 1272/2008.
The REACH Regulation does not define aerosols or aerosol dispensers.
According to the ordinary meaning of the word, an aerosol is considered to be "a substance enclosed under pressure and released as a fine spray by means of a propellant gas". (Oxford advanced dictionary definition). The term may in certain contexts be used for a mixture enclosed under pressure and released as a fine spray by means of a propellant gas, or the dispenser or package used to change the ingredient inside the container into a spray by the use of a propellant gas. The European Aerosol Federation uses the term "aerosol" for both the suspension and the dispenser/package.
Furthermore, it should be noted that according to Article 2.3.1 of the CLP Regulation (for "Classification, Labelling and Packaging"), the term "aerosol dispenser" means: any non-reusable container made of metal, glass or plastic and containing a gas compressed, liquefied or dissolved under pressure, with or without a liquid, paste or powder, and fitted with a release device allowing the contents to be ejected as solid or liquid particles in suspension in a gas, as a foam, paste or powder or in a liquid state.
The CLP definition is very similar to the definition provided in Article 2 of the Aerosol Dispensers Directive (ADD) (75/324/EEC).
The above definitions provide an indication of what could be generally considered as ‘aerosols/aerosol dispensers' in the context of Entry 40 of Annex XVII. Note that aerosol generators should be regarded as aerosol dispensers, as the original restriction discusses aerosol generators.
Entry 40 prohibits the use of flammable, highly flammable or extremely flammable substances in "aerosol dispensers where these aerosol dispensers are intended for supply to the general public for entertainment and decorative purposes". Paragraph 1 provides an indicative list of examples of products that are covered by the restriction. These examples are all products to be used to decorate, for instance, venues for festivities (e.g. Christmas, weddings, and carnivals) or parties (e.g. birthday parties and fancy-dress parties) and for entertainment use, for instance, during festivities and parties. None of the examples listed in the entry are cosmetic products within the meaning of Regulation (EC) No 1223/2009 on cosmetic products.
Coloured hair sprays and body glitter would fall within the definition of cosmetic products in Regulation (EC) No 1223/2009, as they are intended "to be placed in contact with an external part of the human body" with a view to "changing its appearance" and therefore have a similar use to more classical cosmetic products, such as normal hair sprays. Coloured hair sprays and body glitter should not be considered as having an entertainment or decorative purpose, and therefore they are not covered by entry 40 of Annex XVII to REACH.
Through a literature search and consultation with experts in this area it was not found any structural connection between optical brighteners (or better called fluorescent dyes) and azodyes since either the NH bonds in the fluorescent dyes are connected to heterocyclic NC structures and therefore cannot form any of the 22 banned arylamines or they do not contain any azo bonds where reductive cleavage could take place to generate any of the aromatic amines covered by the azodyes ban. Therefore at the present time, this information confirms that the restriction in Entry 43 to Annex XVII does not cover optical brightening agents (OBAs). Should the chemical structure of optical brightening agents be different from the definition as reported above, this answer may change accordingly.
Entry 43(3) of Annex XVII restricts the placing on the market of substances and mixtures containing over 0.1% of the azodyes listed in Appendix 9, when they are intended for colouring textile and leather articles, and also the actual use of the substance or mixture for that purpose. Therefore, the presence of these substances in imported articles is not restricted.
However, pursuant to paragraphs 1 and 2 of the restriction, if an azodye in Appendix 9 releases one or more of the aromatic amines listed in Appendix 8 in a concentration above 30 mg/kg (0,003 % by weight, it cannot be used in textile and leather articles which may come into direct and prolonged contact with human skin or the oral cavity (such as those listed in paragraph 1). Those textile and leather articles cannot be placed on the market unless they comply with that concentration limit.
Yes. The intention of the legislator was to cover all isomers, linear and branched, of nonylphenol and their ethoxylates and therefore all of them are covered by the restriction.
The restriction on nonylphenol and nonylphenol ethoxylates was based on the risks identified in the risk assessment report prepared by the United Kingdom under Council Regulation (EEC) No 793/93 of 23 March 1993 on the evaluation and control of the risks of existing substances. The risk assessment report states that "It is understood that nonylphenol (CAS Number: 25154-52-3) as originally defined by CAS (Chemical Abstract Service) covered all nonylphenols. However, subsequent revisions redefined it to cover only straight chain nonylphenol, other isomers having different CAS numbers. Given the method of manufacture of nonylphenols, very little if any straight chain nonylphenol is produced. That which is produced is only likely to be present at very low levels in commercial mixtures. The commercially produced nonylphenols are predominantly 4-nonylphenol with a varied and undefined degree of branching in the alkyl group. This assessment covers commercially produced material (predominantly 4-nonylphenol, branched). This material will also contain smaller amounts of other isomers and impurities, and falls under the CAS Number 84852-15-3."
Therefore Council Directive 76/769/EEC, as amended by Directive 2003/53/EC, did not specify any CAS or EC numbers for nonylphenol. In the revision of the REACH restriction by Regulation 552/2009/EC, which made several technical changes, the CAS and EC numbers were added. This has been corrected by deletion of the EC and CAS numbers from the entry with the Commission Regulation (EU) 2020/2096.
Paragraphs (1) to (9) of entry 46 list the ‘purposes' to which the restriction applies. Following paragraph (7) "cosmetic products" is paragraph (8) "other personal care products". It therefore seems that cosmetics are to be considered as a subcategory of personal care products.
REACH does not define "cosmetic products". According to Regulation (EC) No 1223/2009 on cosmetic products, "cosmetic product" means "any substance or mixture intended to be placed in contact with the external parts of the human body (epidermis, hair system, nails, lips and external genital organs) or with the teeth and the mucous membranes of the oral cavity with a view exclusively or mainly to cleaning them, perfuming them, changing their appearance protecting them, keeping them in good condition or correcting body odours."
Based on the above, it can be interpreted that "other personal care products" within the meaning of entry 46 include, but are not limited to, any product meeting the conditions indicated in the above definition for substances and mixtures and other products used for personal hygiene, such as toilet papers, some female hygiene products, nappies, cotton pads, etc. This interpretation can be broadly considered as a perception of the average consumer for this product category.
The expression ‘textile articles which can reasonably be expected to be washed in water’ relates to the use of care indications on the label attached to the textile article. Textile articles with care instructions that exclude washing in water and require dry cleaning are, in principle, not covered by the restriction in paragraph 1 of entry 46a.
However, care instructions should not be used to circumvent the restriction; this would for example be the case of a label for dry cleaning where there is no need for dry cleaning. ‘New textile articles produced exclusively from recycled textiles’ means that only recycled textiles materials must have been used to produce new textile articles, otherwise the exemption does not apply. If, for example, virgin textiles materials have been used together with recycled ones, the exemption does not apply.
Entry 48 prohibits the placing on the market for supply to the general public of toluene as a substance or in mixtures, in a concentration equal to or greater than 0.1% by weight, where the substance or the mixture is used in adhesives and spray paints. Adhesive tapes consist of a layer of adhesive coated on a flexible substrate. As the restriction concerns the concentration of toluene in adhesives, the concentration of toluene must be calculated with reference to the amount of adhesive on the tape, and not with reference to the total weight of the adhesive and substrate.
As stated in Recital 8 of the Directive 2005/69/EC (OJ L323, 9.12.2005, p.51), there are at present no harmonized test methods for measuring PAHs in the extender oils, or for measuring PAHs in tyres that contain such oils. Until suitable harmonized methods are available, the only named method that is permitted for measuring the PAH content of extender oils is the IP346 analysis method. This method is permitted providing that certain additional conditions are met. These additional conditions are necessary because the IP346 method does not measure the PAH content directly. In fact IP346 measures the total content of polycyclic aromatic compounds (PCA) rather than the PAH content. The PCAs are a group of substances to which PAHs belong, but in which PAHs are present in only very small amounts. The legal limit for PAHs in extender oils, which is 1 part per million (ppm) of BaP and 10ppm total PAH content, is considered to be met if the total PCA content is <3%. In other words, the PCA content of 3% is taken as a proxy measurement for a PAH content of 10ppm. The proxy measurements will be valid only if the ratio between the PAH and PCA content in the extender oil is known and does not change over time. The additional conditions therefore require an initial calibration of the technique (measurement of the PAH/PCA ratio) and recalibration at intervals of six months, or after "major operational change", in order to ensure that the measurements remain valid over time. The term "major operational change" should therefore be taken to mean any change in materials or processes that could invalidate the results of the proxy measurement. The principle cause of invalid results would be a change in the PAH/PCA ratio in the extender oil. However, it should be remembered that not only is IP346 a proxy method for measuring PAH, but that the quantity that it does measure, namely PCA content, is meaaured in a rather indirect way, namely by a change in the refractive index of a solution, and that PCAs are not the only substances that affect the refractive index of a solution. The potential for obtaining invalid results is therefore quite high and the method should therefore be used with considerable caution. It would therefore be advisable to recalibrate in case of doubt. The provision to control the calibration of the PAH/PCA ratio every six months is to safeguard the validity of the IP346 results against unintentional or unknown changes. This would apply for the case where the manufacturing process and materials used remain the same, and where there is no reason be expect a change in the PAH/PCA ratio. However, it is possible to imagine, for example, that a tyre manufacturer receives a reformulated extender oil from his supplier without being made aware of the change that has been made, and the results from the IP346 could be invalidated as a consequence. A six month recalibration interval was considered sufficient to cover such occurrences. Conclusion: The provision to control the calibration of the PAH/PCA ratio after each "major operational change" is to safeguard the validity of the IP346 results. A major operational change is therefore a deliberate change to materials or processes that might be expected to significantly influence the PAH/PCA ratio, or otherwise affect the validity of the measurement. Examples of such a change would be where the source of supply for the extender oil is changed, or where the method of using the oil is changed. Judgment of whether a particular change is sufficiently important to trigger the need for recalibration will necessarily be made case-by-case and will require expert opinion.
Standard reference tyres are produced and imported solely for the purpose of providing a reference performance for other newly developed tyres. They are not placed on the market to be fitted on vehicles intended for final users. For the purpose of entry 50, tyres are defined as tyres for vehicles covered by Directives 2007/46/EC on motor vehicles and their trailers, Directive 2003/37/EC on agricultural or forestry tractors, and Directive 2002/24/EC on two and three-wheeler motor vehicles. It appears that Reference Tyres are not intended to be used on vehicles covered by the Directives 2007/46/EC, 2003/37/EC and 2002/24/EC. In conclusion these tyres should not be considered as covered by the provisions of the restriction in Entry 50 of Annex XVII.
The threshold of 0.1% is the standard threshold used in Annex XVII. The value of 0.1% has been chosen because it represents a measurable quantity. It is being used to take into account impurities, not to allow the use of certain substances, e.g. phthalates in toys and childcare articles. One should be aware that in order to plasticise a toy or childcare article concentrations of phthalates of more than 10 per cent are needed. Different restrictions are applied to each of the two groups of phthalates. The limit value of 0.1% should therefore be applied for each group of phthalates combined, i.e. the concentration of DEHP, DBP and BBP combined should not be higher than 0.1% and the concentration of DINP, DIDP and DNOP combined should also not be higher than 0.1%. Conclusion: A toy or childcare article would not comply with the Entry 51 or Entry 52 respectively if it contained either more than 0.1% of DEHP, DBP and BBP combined or more than 0.1% of DINP, DIDP and DNOP combined. However, it would be considered compliant if it contained only 0.09% of DEHP, DBP and BBP combined and 0.09% of DINP, DIDP and DNOP combined.
The entries 51 and 52 specify that "Childcare article" means "any product intended to facilitate sleep, relaxation, hygiene, the feeding of children or sucking on the part of children." As these articles are intended to facilitate the hygiene of children they should be considered as "childcare articles" as defined by the entries 51 and 52. In conclusion, articles which are used for the hygienic care of children such as bathtubs, articles for the bath, bathtub mats, hairbrushes, bath thermometers, or nail cutters are therefore covered by the Entries 51 and 52 and use of phthalates and should conform to the prescriptions of the entries.
The definition of childcare articles contained in Annex XVII to REACH is as follows: "Childcare articles" are defined as "any product intended to facilitate sleep, relaxation, hygiene, the feeding of children or sucking on the part of children". Further explanation is provided by the Commission services' guidance document on the interpretation of the concept "which can be placed in the mouth". It gives the following examples: "The main purpose of pyjamas is to dress children when sleeping and not to facilitate sleep. Pyjamas should therefore be regarded as textiles and, like other textiles, do not fall under the scope of the Directive. Sleeping bags are designed to facilitate sleep, and should therefore fall under the Directive." Taking this into account, and also taking into account that the guidance document explicitly contains a description and a photo of a mattress cover, it can be confirmed that mattress protectors are childcare articles as defined in Annex XVII. This means that the three phthalates DEHP, BBP and DBP listed in entry 51 of Annex XVII may not be used in mattress protectors. The other three, DINP, DIDP and DNOP, listed in the entry 52, are only restricted in those articles that can be placed in the mouth by children.
The guidance document contains an example of a mattress cover that is not directly mouthable in normal and foreseeable use conditions. The edges and corner are not accessible for mouthing by the child – by design (the mattress should fit snugly in the cot to avoid entrapment risks), and the mattress is covered with a sheet in normal use and the surface is sufficiently taut (by design – to avoid suffocation risks) to prevent PVC from being mouthed through the sheet. This is based on the observation that inaccessible parts of articles can not be taken into the mouth. Articles or parts of articles should be considered inaccessible if, during proper use or reasonably foreseeable improper use by children, they can not be reached. However, there will be other cases when parts of certain articles can be taken into the mouth under normal and foreseeable conditions, for example when the mattress protector is placed on the sheet or cannot be completely fixed. In conclusion, mattress protectors that can be placed above sheets or that cannot be tightly fixed to the mattress have to comply with the restriction contained in entry 52 of Annex XVII to REACH. Authorities competent for market surveillance should assist manufacturers/importers in making a case-by-case assessment on the basis of the criteria described above and in the guidance document.
The restriction in entry 52 concerns the substance "Di-isodecyl phthalate" (DIDP) which is listed with CAS Numbers 26761-40-0 and 68515-49-1. Di-2-propyl heptyl phthalate (DPHP) is an isomer of decyl phthalate and has the CAS No 53306-54-0. According to the information at the Commission's disposal, DPHP (CAS # 53306-54-0) is different from DIDP and therefore not covered by entry 52 of Annex XVII. In conclusion, the substance is not covered under the entry 52 of Annex XVII. The uses of the substance may be regulated in the future on a Community-wide basis, if it appears from the information which will become available that it causes unacceptable risks to human health or the environment. In addition DPHP is explicitly not promoted by its manufacturers for use in toys, food packaging or medical products.
This guideline aims at providing some criteria and examples to help identify those toys and childcare articles which can be placed in the mouth by children. The guideline lists the main criteria of "size dimension" and "accessible parts" (according to the EN 71-European Standard on the safety of toys) which would therefore facilitate the judgement - on a case by case – whether a toy or childcare article can be placed in the mouth by children. The guideline also provides pictures as examples in order to better indicate which toys and childcare articles or parts of them can be taken into the mouth.
The 0.1% limit was set up in order to eliminate the use of phthalates. Such limit was set up in order to be respected in each bit/piece of plasticised material by itself, regardless of whether there are other pieces or not. Therefore the reference to 0.1%, intended also as the sum up to the 3 phthalates, has to be calculated in each piece of plasticised material of each homogenous material. In the example referred to in the question the calculation should therefore be based on the weight of the plasticised material of the head of the doll and not the entire weight of all plasticised material of the whole toy.
A childcare article is defined by entries 51 (4) and 52(4) as "any product intended to facilitate sleep, relaxation, hygiene, the feeding of children or sucking on the part of children" (see Q&A 983). A baby monitor does not facilitate the sleep or relaxation of the baby, it allows parents to be alerted when the baby is crying or making other noise. Therefore baby monitors fall outside the scope of entries 51-52 of Annex XVII.
The polymeric MDI, with CAS number 9016-87-9, is not included in the definition of the substance with CAS 26447-40-5 and moreover is not classified as dangerous in Annex VI of Regulation (EC) No 1272/2008 (CLP). In the Risk assessment report and Risk reduction strategy performed by the Belgian Rapporteur, two main products were analysed during the exposure assessment and throughout the decision making process: the one-component foams and hot melt adhesives, both containing respectively 10% and 2% of MDI. Other products with MDI content below 0.1% did not pose any risk and therefore were excluded by the final consideration on the risk reduction measures under Directive 76/769/EEC. However, polymeric MDI present in mixtures is covered by the restriction when such mixtures contain more than 0.1% of MDI (as defined in the RAR). Therefore, dimers and polymeric forms of MDI are out of the scope of the current restriction except if they are part of mixtures containing more than 0.1% of MDI.
In entry 58, the terms "including natural or legal persons licensed or authorised in accordance with Council Directive 93/15/EEC" should be read as an example of operators that benefit from the exemption. Therefore the derogation in paragraph 2(a) covers all downstream users and distributors as defined in Article 3(13) and 3(14) of REACH. As a consequence, mixtures containing more than 16% of nitrogen in relation to ammonium nitrate may be placed on the market after 27 June 2010 for supply to downstream users and distributors as defined in REACH. As consumers are not downstream users nor distributors, mixtures containing more than 16% of nitrogen in relation to ammonium nitrate may not be placed on the market for supply to consumers.
If a downstream user uses ammonium nitrate to produce a mixture containing ammonium nitrate below the threshold, it may place this mixture on the market for supply the general public. But downstream users should not use ammonium nitrate in order to produce a mixture containing ammonium nitrate above the threshold for supply to the general public. The restriction is applicable to medical devices as well as to other mixtures containing more than 16% of nitrogen in relation to ammonium nitrate for supply to the general public. For example, instant cold packs which contain more than 16% of nitrogen in relation to ammonium nitrate may not be sold to the general public since 27 June 2010.
The restriction is not applicable to downstream users who use ammonium nitrate for their industrial or professional activities in order to transform it into other substances for different purposes. Therefore, for example the use of ammonium nitrate to produce nitrous oxide for use in the production of pressurised foods or for use as anaesthetics is not restricted.
No. Ink strippers, adhesive removers and degreasing agents are not within the scope of the restriction. The restriction covers paint strippers, including also varnish removers and lacquer removers.
This document echa.europa.eu/documents/10162/17220/lead_guideline_information_en.pdf aims at providing a guideline concerning the interpretation of the scope of the restriction provisions in paragraphs 7 to 10 of entry 63 of Annex XVII to REACH Regulation (EU) No 1907/2006 on lead and its compounds in articles supplied to the general public. It has been drawn up to (i) clarify certain terms that define the scope of the restriction (e.g. "accessible part of articles", "normal/reasonably foreseeable conditions of use") (ii) provide non-exhaustive lists of article types (and examples of sub-types) which fall within (or out of ) the scope of the restriction.
Entry 68 applies to
- Linear and branched perfluorocarboxylic acids of the formula CnF2n +1-C(= O)OH where n = 8, 9, 10, 11, 12, or 13 (C9-C14 PFCAs) including their salts and any combination thereof.
These are the six C9-C14 PFCAs namely:
C9- PFCA - PFNA, EC Nr. 206-801-3 CAS Nr. 375-95-1
C10-PFCA - PFDA, EC Nr. 206-400-3 CAS Nr. 335-76-2
C11-PFCA - PFUnDA EC Nr. 218-165-4 CAS Nr. 2058-94-8
C12-PFCA - PFDoDA EC Nr. 206-203-2 CAS Nr. 307-55-1
C13-PFCA - PFTrDA EC Nr. 276-745-2 CAS Nr. 72629-94-8
C14-PFCA - PFTeDA EC Nr. 206-803-4 CAS Nr. 376-06-7
and their salts,
- Any C9-C14 PFCA-related substance having a perfluoro group with the formula CnF2n +1- directly attached to another carbon atom, where n = 8, 9, 10, 11, 12, or 13, including their salts and any combinations thereof.
These are the related substances which can degrade or be transformed to the C9-14 acids. PFCA-related substances are substances that, based upon their structural formulae, are considered to have the potential to degrade or be transformed to C9-14 perfluorocarboxylic acids (linear and/or branched).
- e.g. Ammonium nonadecafluorodecanoate
and 2,2,3,3,4,4,5,5,6,6,7,7,8,8,9,9,10,10,11,12,12,12-docosafluoro-11-(trifluoromethyl)- dodecanoyl fluoride
however, note that the substance methyl nonacosafluoropentadecanoate has a perfluoro group CnF2n+1- directly attached to another carbon atom however the perfluorinated chain (C14F29-) does not fall within the range n=8, 9, 10, 11, 12 or 13 and is outside the scope of the restriction. The nomenclature CnF2n+1- means any branched or linear perfluorinated alkyl moiety containing carbon atoms on which all the H substituents have been replaced by F atoms. The `-´ represents ‘bonded to’ and any group can be bonded to the CnF2n+1- moiety, including for example iodine. If CnF2n+1- is directly attached to another carbon atom it is out of scope.
3. Any C9-C14 PFCA-related substance having a perfluoro group with the formula CnF2n +1- that it is not directly attached to another carbon atom, where n = 9, 10, 11, 12, 13 or 14 as one of the structural elements, including their salts and any combinations thereof.
An Example of this group is: 1,1,1,2,2,3,3,4,4,5,5,6,6,7,7,8,8,9,9,10,10,11,11-tricosafluoro-11-iodo-undecane:
The following substances are excluded:
4. Substances with formula CnF2n +1-X, where X = F, Cl, or Br
where n = 9, 10, 11, 12, 13 or 14, including any combinations thereof.
These are perfluorinated substances with a halogen attached and as such are a different group of substances that are not degraded to PFCAs. Iodine is not excluded by this group because it is the starting point for the telomerisation process.
5. Substances with formula CnF2n +1-C(= O)OX' where n> 13 and X'=any group, including salts.
The nomenclature X’ means any possible functional group.
This exclusion is for substances that contain perfluoro groups having higher carbon numbers than those mentioned under paragraph 1 and are as such not covered by the restriction because only C9-C14 PFCAs have been identified as PBT or vPvB substances.
Both linear and branched C9-C14 perfluorocarboxylic acids are included in the scope of restriction entry 68. Polyfluorinated (i.e. partially fluorinated) substances containing a structural element with a sufficiently long perfluorinated moiety are included within the scope of the restriction because they degrade to perfluorinated (fully fluorinated) C9-C14 PFCAs e.g. 8:2 FTOH:
Polyfluorinated substances containing other partially fluorinated structural elements such as the substance below are not included within the scope of the restriction because they do not contain a structural element with a sufficiently long perfluorinated moiety (e.g., 2,2,3,3,4,4,5,6,6,7,7,8,8,9,9,9-hexadecafluorononyl methacrylate:
It should be noted that if a substance contains structural elements both inside and out of scope, then the substance is still within the scope.
More information is available in the Final background document accessible on the ECHA website in the Registry of restriction intentions until outcome section.
This guideline clarifies how users of NMP can ensure their compliance with the derived no-effect levels (DNELs) established in the restriction on 1-methyl-2-pyrrolidone (NMP) in entry 71 of Annex XVII to the REACH Regulation (EU) No 1907/2006. The guideline explains how to proceed when there are mandatory DNELs in addition to the occupational exposure limit values (OELs). It clarifies what the user needs to do to adequately control the risks. Several examples of good practice illustrate the type of equipment some companies have in place to control inhalation and dermal exposure in various tasks such as transfers, maintenance and sampling. In addition, examples of monitoring methods, including biological monitoring methods, are described. The document has been prepared in close cooperation with the industry stakeholders and endorsed by the Member States authorities.
In your language:
The use of NMP (as such or in a mixture) during the production of articles is covered by the restriction. This means that you have to apply the conditions set by the restriction in your production operations. More information on how to do that is available in the NMP guideline.
The use of articles containing NMP is not considered as use of the substance (as such or in a mixture), and thus is not subject to the restriction. This means that your customers using the article you produce do not need to comply with the conditions set by the restriction.
However, it is important to note that NMP is listed in the Candidate List and specific obligations apply to its presence in articles. Similar duties are also set in the revised Waste Framework Directive 2008/98/EC. For more information see SCIP database.
Last but not least, if you are not sure how to differentiate articles from substances or mixtures, the ECHA guidance on requirements for substances in articles, Appendix 4 will help you.
Appendix 12 of Annex XVII which is referred to in entry 72 includes five phthalates, i.e. 1,2-benzenedicarboxylic acid; di-C 6-8-branched alkylesters, C 7-rich, bis(2-methoxyethyl) phthalate, diisopentylphthalate, di-n-pentyl phthalate (DPP) and di-n-hexyl phthalate (DnHP).
Clothing and related accessories, textiles other than clothing which under normal or reasonably foreseeable conditions of use come into contact with human skin to an extent similar to clothing and footwear cannot contain a total concentration equal or greater than 1000 mg/kg of the phthalates specified in the Appendix 12, considered individually or in combination with other phthalates listed either in i) Appendix 12, or ii) elsewhere in Annex XVII, if classified as carcinogenic, mutagenic or toxic to reproduction (CMR), category 1A or 1B in Part 3 of Annex VI to Regulation (EC) No 1272/2008.
Examples of other phthalates in Annex XVII include those listed in entry 51 (bis (2-ethylhexyl) phthalate (DEHP), benzyl butyl phthalate (BBP), dibutyl phthalate (DBP) and diisobutyl phthalate (DIBP)). These substances are classified as toxic to reproduction category 1B, and therefore fulfil the criteria of the restriction. Thus they cannot be present in the clothing and related accessories, other textiles and footwear placed on the market if, in combination with the phthalates listed in Appendix 12, the total phthalate concentration, measured in homogeneous material, is equal to or greater than 1000 mg/kg. Note also that Appendices to entries 28 to 30 provide lists of substances classified as CMRs, category 1A or 1B to which the restrictions apply. The above considerations are relevant also to the phthalates included in those Appendices.
Restriction entry 73 concerns ‘(3,3,4,4,5,5,6,6,7,7,8,8,8-tridecafluorooctyl) silanetriol and any of its mono-, di- or tri-O-(alkyl) derivatives (hereafter referred as TDFAs)’
The restriction targets spray products for supply to the general public (e.g. aerosol dispensers, pump and trigger sprays and mixtures marketed for spray application) containing mixtures of TDFAs and organic solvents.
A general formula for the targeted group of substances can be given as:

In theory, variations in the alkoxy chain can give rise to a very large number of parent compounds. It is important to note, however, that the restriction applies to all substances which are covered by the general formula in order to avoid substitution to other polyfluorooctyl alkoxysilanes.
Examples of substances targeted by the restriction:
More information is available in the Final background document accessible on the ECHA website in the Registry of restriction intentions until outcome section.
Restriction entry 74 applies to ‘Diisocyanates, with generic formula O=C=N-R-N=C=O, with R being an aliphatic or aromatic hydrocarbon unit of unspecified length’. This means that the group R in this entry does not contain urethane, urea, uretdione, biuret, allophanate or isocyanurate linkages (i.e., the diisocyanate entity is not the result of prepolymerisation of a parent diisocyanate). The restriction covers industrial and professional uses of Diisocyanates (as substances on their own, as constituents of other substances or in mixtures) in concentration ≥0.1% by weight. As a result, oligomers and prepolymers that contain ≥0.1% (by weight) of the diisocyanates that meet the above definition (of which a non-exhaustive list is shown below), would still be in scope of the restriction.
Examples of diisocyanate substances covered by restriction entry 74:

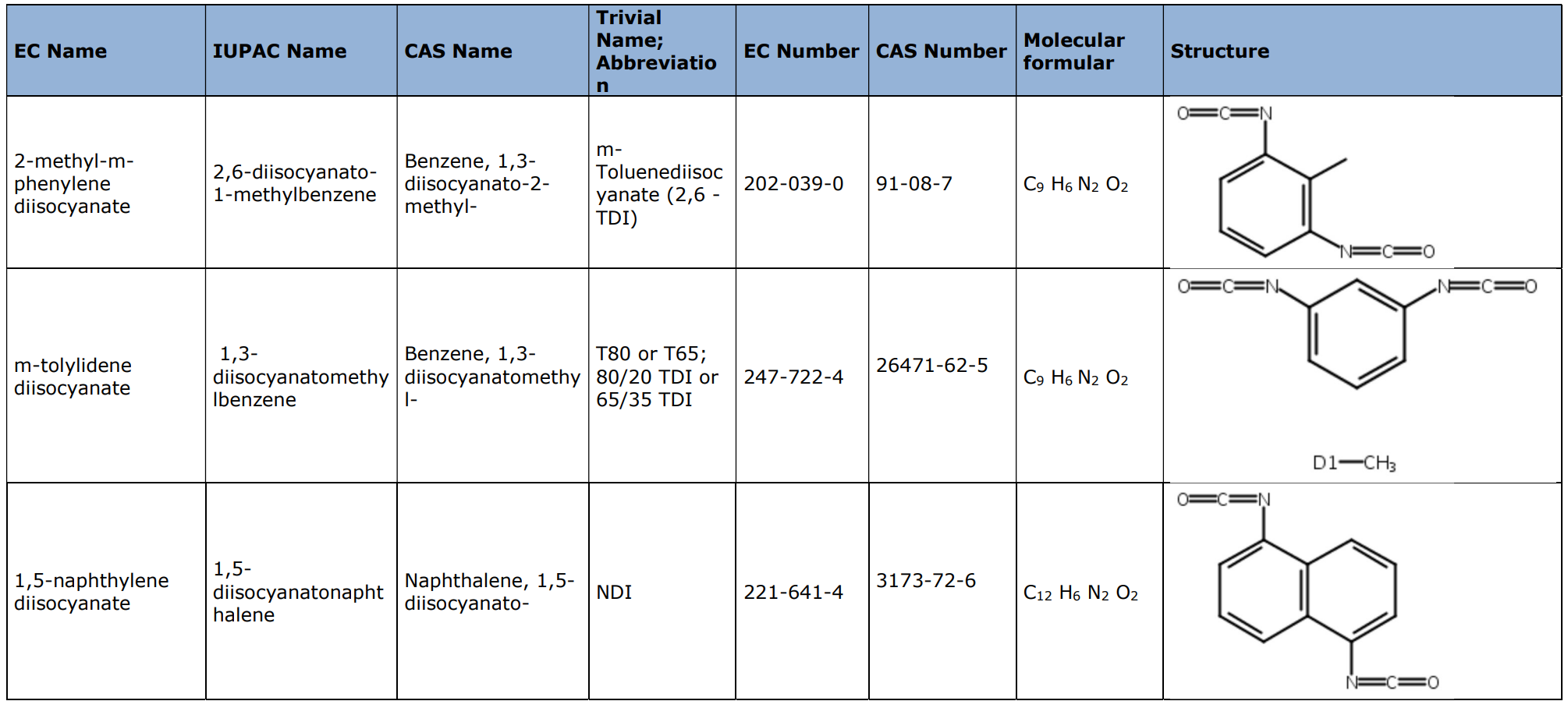
More information is available in the Final background document accessible on the ECHA website in the Registry of restriction intentions until outcome section.
Paragraph 4 of the restrictions indicates that the “training shall be conducted by an expert on occupational safety and health with competence acquired by relevant vocational training”. Restriction entry 74 does not contain additional education requirements (e.g., University degree on Chemistry or Toxicology) for trainers. Trainers must be able to demonstrate their qualification as expert on occupational safety and health and, in addition, to have acquired specific competence in the fields relevant for the restriction through vocational trainings. The qualifications of the experts should comply with specific provisions on educational and professional requirements of experts on occupational safety and health as required by the applicable legislation, including those established by the Member States.
There are no provisions in restriction entry 74 on how to deliver the training, therefore it can be assumed that the content can be provided also online through pre-prepared recorded and/or interactive training sessions. However, a qualified trainer (as described above) is needed to provide responses to possible questions by participants during or after the training and to verify and validate possible final tests aiming to verify if the content of the training has been understood by participants. It should be considered that the scope of the restriction is to ensure that workers handling diisocyanates have a thorough understanding of the risks and of the health and safety measures to adopt to control them (including in emergency situations).
As outlined in paragraph 1 of the restriction entry 74 of Annex XVII to REACH,
Diisocyanates,
1. Shall not be used as substances on their own, as a constituent in other substances or in mixtures for industrial and professional use(s) after 24 August 2023, unless:
a) the concentration of diisocyanates individually and in combination is less than 0,1 % by weight, or
b) the employer or self-employed ensures that industrial or professional user(s) have successfully completed training on the safe use of diisocyanates prior to the use of the substance(s) or mixture(s).
It is further outlined in paragraph 5 of the restriction that:
The training referred to in point (b) of paragraph 1 shall include the instructions for the control of dermal and inhalation exposure to diisocyanates at the workplace without prejudice to any national occupational exposure limit value or other appropriate risk management measures at national level. Such training shall be conducted by an expert on occupational safety and health with competence acquired by relevant vocational training.
That training shall cover as a minimum:
a) the training elements in point (a) of paragraph 5 for all industrial and professional
use(s).
b) the training elements in points (a) and (b) of paragraph 5 for the following uses: handling open mixtures at ambient temperature (including foam tunnels), spraying in a ventilated booth, application by roller, application by brush, application by dipping and pouring, mechanical post treatment (e.g. cutting) of not fully cured articles which are not warm anymore, cleaning and waste, any other uses with similar exposure through the dermal and/or inhalation route.
c) the training elements in points (a), (b) and (c) of paragraph 5 for the following uses: handling incompletely cured articles (e.g. freshly cured, still warm), foundry applications, maintenance and repair that needs access to equipment, open handling of warm or hot formulations (> 45 °C), spraying in open air, with limited or only natural ventilation (includes large industry working halls) and spraying with high energy (e.g. foams, elastomers and any other uses with similar exposure through the dermal and/or inhalation route.
The text of the restriction states that the employer (or self-employed worker) has full responsibility that industrial and professional workers have successfully completed a training on the safe use of diisocyanates before they use these substances (or mixtures).
Suppliers shall ensure that the recipients of substance(s) or mixture (s) are provided with the training materials and courses (paragraph 7). The restriction does not specify who has to prepare that material, but the material provided by the supplier must comply with paragraphs 4 and 5 of the entry and take into consideration the specificity of the products supplied, including composition, packaging, and design. Therefore, input of the manufacturer/producer may be relevant.
Furthermore, the restriction indicates that the training has to be conducted by an expert on occupational safety and health with competence acquired by relevant vocational training. Minimum requirements for the training are also indicated in the entry.
The employer is responsible to select the training provider, who is responsible to provide the training covering as a minimum the elements laid down in paragraph 5 of the restriction. The employer will have also the duty to ensure that the provider is qualified to conduct the training and to verify that the training material provided by the supplier covers as a minimum the topics indicated in points a) and b) and, where applicable c), of paragraph 5 of the restriction and that the training effectively provided by the expert covers such topics.
The restriction entry 74 sets conditions on the use of diisocyanates as substances on their own, as a constituent in other substances or in mixtures for industrial and professional use as well as on placing them on the EU/EEA market. The restriction entry is available in the document ANNEX XVII to REACH – Conditions of restriction.
The restriction does not apply to articles as defined under REACH. For more information, see the ‘Guidance on requirements for substances in articles’.
The supplier of the substance or mixture is responsible for providing the required information and that the packaging contains the required statement as per paragraph 2(b) of restriction entry 74 of Annex XVII to REACH.
Under the REACH regulation, a supplier is defined as “any manufacturer, importer, downstream user or distributor placing on the market a substance, on its own or in a mixture, or a mixture” (Art. 3(32) of REACH). Furthermore, ‘placing on the market’ means supplying or making available, whether in return for payment or free of charge, to a third party. Import is deemed to be placing on the market (Art. 3(12) of REACH). Therefore, any supplier, including the manufacturer of these substances, the formulator of mixtures containing these substances and a distributor down the supply chain, needs to ensure the provision of the correct information and required statement on the packaging of the substance or mixture they place on the market, even if the substance or mixture was manufactured or formulated before 24 February 2022.
Restriction entry 75 of Annex XVII to REACH covers different categories of substances that are considered to pose risks to human health if they are used for tattooing purposes. The substances restricted for use in tattoo inks and permanent make up include:
1. Substances with the following harmonised classifications:
- carcinogenic and mutagenic (CM), categories 1A, 1B and 2 if present in mixtures for use for tattooing purposes in a concentration ≥0,00005 % by weight
- reproductive toxicants (repro), categories 1A, 1B and 2 and skin sensitisers (SS) Cat. 1, Cat. 1A and Cat. 1B if present in mixtures for use for tattooing purposes in a concentration ≥0,001 % by weight
- Substances with harmonised classification as skin irritant (Cat. 2), skin corrosive (Cat. 1, Cat. 1A, 1B, 1C), eye irritant (Cat. 2) or serious eye damaging (Cat. 1) if present in mixtures for use for tattooing purposes in concentration ≥ 0,1 % by weight (if the substance is used only as a pH regulator) or ≥ 0,01 % by weight, in all other cases;
NOTE: substances with harmonised classification as carcinogen, mutagen and reproductive toxicant category 1A, 1B or 2 for effects due only to inhalation exposure are excluded.
2. Substances in the scope of Regulation (EU) 1223/2009 - Cosmetic Products Regulation (CPR) – present in mixtures for use for tattooing purposes in concentration ≥0,00005 % by weight i.e.:
- Substances listed in Annex II of CPR Regulation (list of substances prohibited in cosmetic products)
- Substances listed in Annex IV of CPR Regulation
- Restricted due to condition of use of one or more of this kind specified in column g of Annex IV: “Rinse-off products”; “Not to be used in products applied on mucous membranes”; “Not to be used in eye products”
- the substance is present in the mixture in a concentration, or in some other way, that does not accord with the conditions specified in column h or i of Annex IV.
3. Substances included in Appendix 13 of Annex XVII to REACH
The REACH restriction does not contain a positive list of substances that can be used in tattoo inks and permanent make-up. Substances not included in the categories indicated in restriction entry 75 of Annex XVII to REACH above the limits specified therein or in any other applicable entry (e.g., entries 28-30 ban the placing on the market, or use, for supply to the general public of substances with harmonised classification as carcinogen, germ cell mutagen or reproductive toxicant, categories 1A or 1B listed in appendices 1 to 6 of Annex XVII to REACH) are allowed to be used in tattoos.
The following substances are excluded from restriction entry 75:
1. Substances classified as carcinogens or mutagens in Categories 1A, 1B and 2 only with the hazard statements H350i (May cause cancer by inhalation), H351i (Suspected of causing cancer by inhalation), H340i (May cause genetic defects via inhalation) and H341i (Suspected of causing genetic defects by inhalation)
2. Substances classified for reproductive toxicity (repro) categories 1A, 1B and 2 only with the hazard statement H340(inhalation) (May cause genetic defects via inhalation) and H341(inhalation) (Suspected of causing genetic defects by inhalation)
3. Substances that are gases at temperature of 20 °C and pressure of 101,3 kPa, or generate a vapour pressure of more than 300 kPa at temperature of 50 °C., with the exception of formaldehyde (CAS No 50-00-0, EC No 200-001-8)
The following substances can still be used in tattoos until 4th of January 2023:
- Pigment Blue 15:3 (CI 74160, EC No 205-685-1, CAS No 147-14-8);
- Pigment Green 7 (CI 74260, EC No 215-524-7, CAS No 1328-53-6
Mixtures placed on the market for use for tattooing purposes need to be marketed with the statement ‘Mixture for use in tattoos or permanent make-up’. The mixture must contain information indicated in Paragraph 7 of the restriction entry.
More information is available in the Final background document accessible on the ECHA website in the Registry of restriction intentions until outcome section.
Operators in the EU/EEA need to comply with the conditions specified in any relevant restriction entry that applies to substances placed on the market in a product or used in the EU/EEA market.
This means that manufacturers, importers and downstream Users (DU) of the substances listed in the restrictions must comply with the provisions of Entry 75. For instance, a tattoo artist who is formulating a mixture needs to comply with the relevant obligations of this restriction as he/she is considered as a downstream user. For further details, see Q&A 171.
The enforcement of REACH restrictions is a responsibility of each EU Member State, as well as the members of the EEA (Norway, Iceland and Liechtenstein). They must ensure that there is a system of official controls and lay down legislation specifying penalties for non-compliance with the provisions of REACH. For further details, see Q&A 3.
Many substances have multiple harmonised classifications and some of them are also included into Annex II or Annex IV of the CPR Regulation. Concentration limits specified in entry 75 of annex XVII to REACH may differ for different category of substances. If a substance falls into more than one of the categories of substances restricted under Entry 75 of Annex XVII to REACH, the lowest of the concentration limits applicable to each category has to be taken into account.
As an example, formaldehyde has a harmonised classification as Carc 1B, Muta 2 , and a harmonised classification as Skin Sens. 1. The following concentration limits are established in entry 75:
- 0,00005 % by weight for substances with harmonised classification as Carc 1 B ad Muta 2
- 0,001 % by weight for substances with harmonised classification as Skins sens 1.
In the case of Formaldehyde the limit of 0,00005% by weight applies in light of its classification as Carc 1B/Muta 2.
Restriction entry 75 applies from the date of the entry into application of the harmonised classification for substances that receive a Harmonised Classification which falls into the scope of the entry (i.e. CM 1A, 1B or 2 Repro 1A, 1B or 2, Skin Sens 1, 1A or 1B, Skin Corr. 1, 1A, 1B or 1C, Skin Irritant 2, serious eye damage category 1 or eye irritant category 2.
If a substance is included into Annex II or Annex IV of the CPR Restriction entry 75 applies 18 months from the date of inclusion in the relevant Annex.
Information on suitable analytical methods to verify compliance to the provisions in entry 75 is provided in the Annex to the Background Document, Appendix D.2. However, national enforcement authorities of Member States may have developed additional test methods for tattoo inks.


