- ECHA
- Législation
- BPR
- Approbation des substances actives
- Substance active existante
- Evaluation process
Evaluation process for active substances under the review programme
Evaluation process for active substances under the review programme
Active substances already in the review programme (Annex II part 1) and active substances which will be added to the programme after the submission of a compliant notification will be evaluated as described below.
The evaluating competent authority has 365 days from the validation date or from the time limits provided for by Annex III of the Review Programme Regulation, whichever is the latest, to assess the application and provide its assessment report and conclusions to ECHA.
Applications are submitted through R4BP 3 as a IUCLID file.
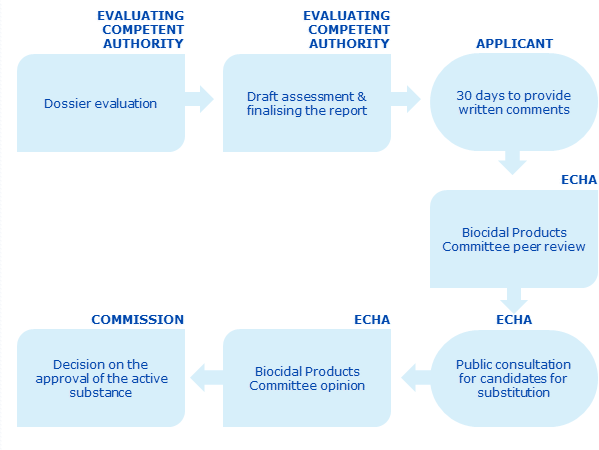
This graphic shows an overview of the dossier evaluation process
Steps
During the evaluation process, the applicant may be requested to provide additional information if the evaluating competent authority considers that more information is necessary. The participant has to provide the requested information within a specified time limit, which will be a maximum 365 days in cases where the additional information relates to concerns which were not addressed under Directive 98/8/EC or 180 days in all other cases. These time-limits cannot be exceeded unless a delay is justified by the nature of the data requested or by exceptional circumstances.
The evaluation process has the following steps:
The evaluating competent authority carries out the dossier evaluation.
The evaluating competent authority finalises the draft assessment report and the conclusions of its evaluation.
The draft assessment report is sent to the participant(s) through R4BP 3. The applicant has 30 days to provide written comments. Where several participants support the same substance/product-type combination, the evaluating competent authority shall draft only one assessment report.
The assessment report is transmitted through R4BP 3 to ECHA for peer review in the Biocidal Products Committee (BPC).

If the active substance is a candidate for substitution, a public consultation is launched. This gives third parties the possibility to submit relevant information, including information on alternative substances.
The BPC has 270 days to deliver an opinion through a peer review assessment and to submit this opinion on approval of the substance/PT combination or its inclusion in category 1, 2, 3, 4, 5 or 6 of Annex I of the BPR, or both, to the Commission.
The preparation of the opinion starts either within three months from the acceptance of the assessment report or by the time limits provided for by Annex III whichever is the later.

Following a positive decision taken by the Commission, the active substance is approved and included on the Union list of approved active substances or included in the Annex I of the BPR.
Actors
The main actors in the evaluation process are:
Participants
Participants are responsible for providing all necessary information in their dossiers. They should pay attention to the various deadlines throughout the process. Applicants have the possibility to comment on the draft assessment report of their dossier during the process.
Public
If the active substance is a candidate for substitution, interested parties have the possibility to submit relevant information during the public consultation procedure.
ECHA
ECHA coordinates the approval process and provides the necessary tools and support for the participants. ECHA also provides the Secretariat for the Biocidal Products Committee.
Biocidal Products Committee (BPC)
The Biocidal Products Committee gives a scientific opinion on active substances (approval, renewal, review, inclusion in Annex I), Union authorisation of biocidal products and mutual recognition. The Committee also deals with scientific and technical matters and other questions at the request of the European Commission and the Member States. The Committee consists of members appointed by EU Member States and EEA countries on the basis of their experience
Evaluating competent authorities
The evaluating competent authority is responsible for carrying out the evaluation of the applications.
European Commission
The European Commission, assisted by the Standing Committee on biocidal products, takes into consideration the opinion issued by the BPC and decides whether to approve the active substance or not. The Standing Committee is chaired by the Commission and has representatives from all Member States. Following a positive decision, the Commission includes the active substance on the Union list of approved active substances or in the Annex I of the BPR.