- ECHA
- Législation
- REACH
- Cooperation with authorities and stakeholders
- Groupe d'experts sur les substances persistantes, bioaccumulables et toxiques (PBT)
- Management of PBT/vPvB substances under REACH
Management of PBT/vPvB substances under REACH
-
Cooperation with authorities and stakeholders
- RIME+ Platform
- Groupe d'experts sur les substances persistantes, bioaccumulables et toxiques (PBT)
- Groupe d'experts sur les perturbateurs endocriniens
- REACH Exposure Expert Group
- Groupe de travail PETCO
- Plastic additives initiative
- Metals and Inorganics Sectoral Approach
- ECHA-CEFIC collaboration on dossier compliance
Management of PBT/vPvB substances under REACH
The REACH Regulation pays specific attention to the PBT/vPvB concern. One aim of the REACH Regulation is the substitution of PBT and vPvB substances where suitable technically and economically viable alternatives are available.
Until the substitution is achieved, the releases of and exposures to PBT and vPvB substances need to be minimised. Various tasks stipulated in the REACH Regulation to achieve this aim are carried out by the registrants, authorities in the EU Member States, ECHA and the European Commission. These are:
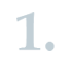
The registrants of substances, manufacturing or importing in quantities equal to or more than 10 tonnes per year, conduct a PBT/vPvB assessment in accordance with Section 4 of Annex I and Annex XIII of the REACH Regulation. ECHA guidance provides support on how to carry out this assessment. If the registrants conclude that their substance fulfils the PBT or vPvB criteria, they are obliged to identify risk management measures and operational conditions (i.e. exposure scenarios) that minimise the releases and exposures in the whole life-cycle of the substance. The registrants have to implement such measures on their site(s) and communicate the relevant measures to their downstream users. The downstream users have an obligation to implement the measures on their site(s) and pass on the necessary information further in the supply chain.
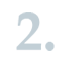
Through dossier evaluation, ECHA can make sure that the registrants' PBT/vPvB assessments comply with the legal and scientific standards required by the REACH Regulation.
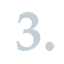
EU Member State competent authorities may carry out substance evaluation for prioritised substances on the Community rolling action plan, which are suspected to potentially be PBT or vPvB substances in order to clarify whether the substances actually fulfil the PBT/vPvB criteria. ECHA coordinates the related decision-making process.
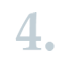
EU Member State competent authorities and ECHA (at the request of the European Commission) may suggest substances to be identified as substances of very high concern (SVHCs) in accordance with Article 59 of the REACH Regulation because they fulfil the PBT/vPvB criteria. In such cases, the authority needs to provide information justifying that the substance fulfils the criteria set out in Annex XIII of REACH.

The inclusion of the substance in the Candidate List due to its PBT/vPvB properties immediately triggers the obligation of registrants to recognise, if not already done, this status in their registration dossiers and in their supply chain communication. The registrants and downstream users must then implement the necessary measures for minimising the releases and exposures in the whole supply chain and lifecycle. The generic obligations triggered by the Candidate List inclusion also apply.
PBT/vPvB substances included in the Candidate List will be subject to prioritisation and will eventually be included into Annex XIV to the REACH Regulation (the Authorisation List). The aim of the authorisation requirement is to make sure at the Community level that the risks arising from the uses of PBT/vPvB substances are properly controlled and that these substances are progressively replaced with suitable alternative substances or technologies where these are economically and technically viable. Alternatively, if more suitable, restrictions under Title VIII of REACH may be developed.
Related links
Related documents
- Weight of Evidence in PBT assessment-Examples [PDF] [EN]
- Mammalian toxicokinetic database (MamTKDB) [XLSX] [EN]
- Critical literature review of analytical methods applicable for environmental fate studies [PDF] [EN]
- Background note: addressing non-extractable residues in regulatory persistence assessment [PDF] [EN]
- Guidance on information requirements and chemical safety assessment Chapter R.11: PBT Assessment [PDF] [EN]
- Bioaccumulation assessment of air-breathing mammals: a discussion paper [PDF] [EN]
- Sterile controls in biodegradation studies – current status in regulatory testing in persistence assessment under REACH [PDF] [EN]
- Options to assess persistence of volatile substances in regulatory PBT assessment [PDF] [EN]


