Dossier submission for active substance approvals or inclusion in Annex I to BPR
Dossier submission for active substance approvals or inclusion in Annex I to BPR
An application for the approval of an active substance (inclusion in the Union list of approved active substances) or for the inclusion in Annex I of the BPR (with the exclusion of category 7) can be submitted only by a participant whose notification has been found compliant by ECHA.
Applications for the active substances listed in Annex II part 1 of the Review Programme Regulation (Regulation (EU) No 1062/2014) have already been submitted.
The applications for additional substance/ product-type combinations (Article 15) or where a new entity takes over the role of participant (Article 14) must be submitted within two years from the declaration of compliance of the notification issued by ECHA.
Applications are submitted through R4BP 3 as a IUCLID file.
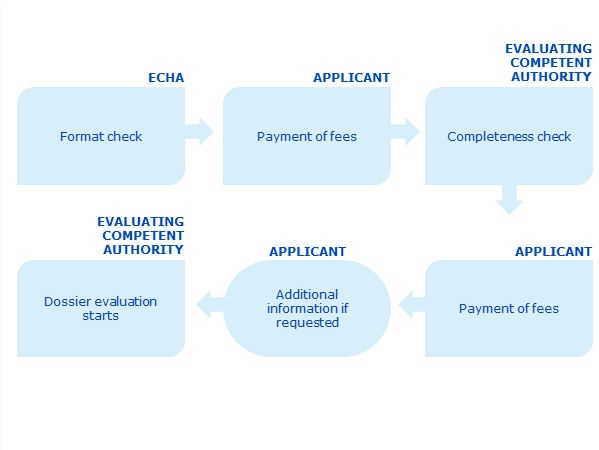
This graph shows an overview of the dossier submission process
Steps
Dossier submission proceeds in several steps. Each step needs to be completed before the application can move to the next step. It is important that the participant makes sure that all applicable deadlines are met otherwise the application will be rejected during the course of the process.
ECHA checks that the application and data have been submitted in the correct format and inform the applicant of the applicable fees.
The participant pays the related fees to ECHA within 30 days from the invoice date. The application will be rejected if the participant does not pay the ECHA fee within the deadline. In case of rejection, an appeal may be brought, in accordance with Article 77 of the BPR.
ECHA accepts the application and informs the participant and the evaluating competent authority which has 30 days to inform the participant of the applicable fees.
The participant pays the related fees to the evaluating competent authority within 30 days.

The evaluating competent authority validates the application for approval or inclusion in category 6 of Annex I of the BPR within 30 days of the payment of the fees. For an application for inclusion in category 1, 2, 3, 4 or 5 of Annex I to BPR the validation is considered to be finalised when the application is accepted by ECHA and the participant pays the relevant fee to the evaluating competent authority.
If the dossier is deemed to be incomplete, the evaluating competent authority will ask for the missing information and the applicant will have a reasonable time limit (normally a maximum of 90 days) to provide it.
The evaluating competent authority must validate the application within 30 days of receipt of the additional information, if the additional information is sufficient

Evaluation of the dossier starts.
Actors
The main actors in the dossier submission process are:
Participants
Participants have the responsibility to provide dossiers with all relevant information on their active substances and to provide additional information if requested by the evaluating competent authority. Participants are responsible for the quality of the data in their dossiers.
ECHA
ECHA is responsible for ensuring that the information in the dossiers is in a correct format. ECHA also makes sure that the submission process proceeds within the set timelines.
Evaluating competent authority
The evaluating competent authority is responsible for carrying out the validation of the received dossiers and subsequently for the evaluation of the dossiers submitted by the participants. Unless the Members State/ evaluating competent authority is already mentioned in Annex II to the Review Programme Regulation, the participants can choose which Member State they would like to evaluate their dossiers, in which case written confirmation that the chosen evaluating competent authority has agreed to do so must be submitted.