- ECHA
- Support
- Guidance
- Guidance Documents
- Guidance on Information Requirements and Chemical Safety Assessment
Guidance on Information Requirements and Chemical Safety Assessment
Guidance on Information Requirements and Chemical Safety Assessment
PATHFINDER
This guidance describes the information requirements under REACH with regard to substance properties, exposure, use and risk management measures, in the context of the chemical safety assessment. It is part of a series of guidance documents that are aim to help all stakeholders with their preparation for fulfilling their obligations under the REACH Regulation.
The Guidance covers:
- the collection of available information regarding the intrinsic properties of substances to be registered
- the assessment of this information against the requirements specified by REACH,
- the identification of data gaps and
- the generation of the additional information required to fill the data gaps.
The Guidance also aims to assist industry in conducting Chemical Safety Assessments and preparing Chemical Safety Reports, when required. A CSR may be required as part of a registration dossier (for non intermediates > 10 t/a), as part of an authorisation application, or as part of downstream user obligations. It also sets out the basic principles for authorities preparing a risk assessment. This may be needed in support of a restriction proposal, of a proposal to include substances into the authorisation regime, or as part of a Substance Evaluation.
The Guidance consists of two major parts: Concise guidance (Part A to F) and supporting reference guidance (Chapters R.2 to R.20).
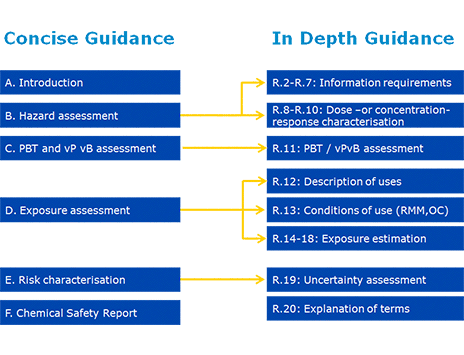
Figure 1: Structure of the Guidance
The purpose of the concise guidance is to support the processes needed to meet the information requirements on intrinsic properties of substances to be registered, and where relevant to carry out a chemicals safety assessment. This includes information collection processes, communication processes and assessment processes. The purpose of the reference guidance is to provide in-depth scientific and technical advice. The links between the concise guidance and the supporting reference guidance are illustrated in Figure 1.
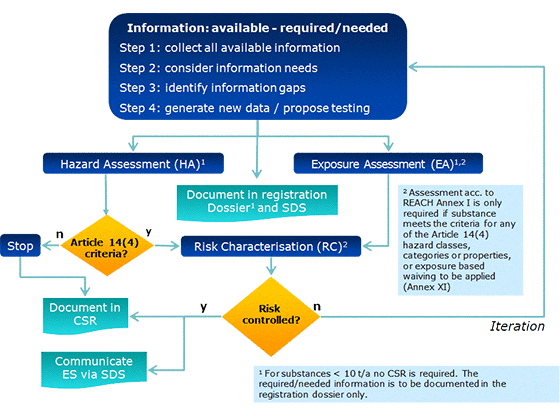
Figure 2: Overall process related to information requirements and chemicals safety assessment under REACH.
Figure 2 above presents an overview of the overall process of collecting and assessing existing information on the intrinsic properties of a substance, including identification of needs to generate new data. It also describes the process of chemical safety assessment additionally required for substances produced/imported in amounts of more than 10t per year.
Figure 3 below illustrates to which steps in the overall process a particular guidance element is related.
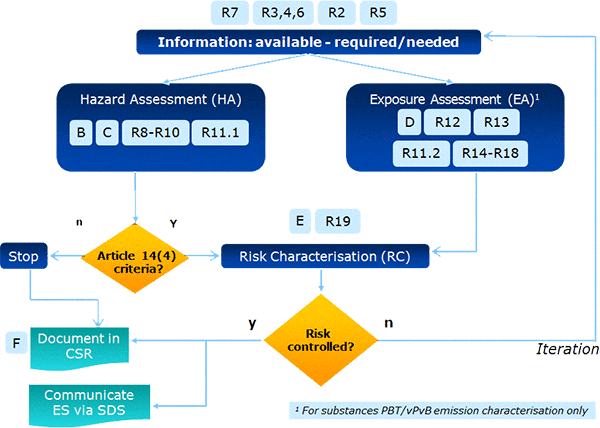
Figure 3: Relationship between the process steps and the guidance elements
Structure of the Guidance on Information Requirements and Chemical Safety Assessment
Part A provides an introduction to the guidance for conducting the chemical safety assessment and preparing the chemical safety report for substances manufactured or imported in a quantity of 10 tonnes or more per year (Chapter A.1). This includes an overview on the intended outcomes and main contents of the chemicals safety assessment (CSA). It also includes the overall approach to making cost-effective decisions during the iterative process of conducting the CSA, and a pathfinder to the different elements of this guidance. Chapter A.2 explains the key facts needed to understand the process of making a chemical safety assessment. The communication and tasks within the supply chain related to the chemical safety assessment are outlined in Chapter A.3. Chapter A.4 describes in more detail, under what conditions an actor may need to carry out a CSA under REACH.
- Part A - Introduction to the Guidance Document (9/12/2011)
Part B contains concise guidance on hazard assessment. This covers information requirements on intrinsic properties of a substance under REACH, including information gathering, non-testing approaches and the so-called 'integrated testing strategies' for generating the relevant and required information on each endpoint. Part B also provides concise guidance on how to characterise hazards, including where possible derivation of DNELs and PNECs. Each of the sections in Part B corresponds to the more in-depth guidance contained in Chapters R.2 to R.10.
This includes:
- Physico-chemical properties in Section R.7.1
- Determination of Derived No-Effect-Levels (DNEL) (or other qualitative or semi-quantitative measures of potency of the substance) in Chapter R.8 and the corresponding chapters of integrated testing strategies for the relevant human health endpoints (Sections R 7.2 to R.7.7 in Chapter 7a). These Sections in Chapter R.7 also include information on how to derive appropriate information for classification and labelling of the substance. However, the guidance on classification and labelling itself is provided elsewhere. See current Annex VI to Directive 67/548 and future Guidance on Classification, Packaging and Labelling related to the future GHS system.
- Determination of Predicted No-effect-Effect levels (PNEC) in Chapter R.10 and the corresponding chapters of integrated testing strategies for the environment endpoints (Sections R.7.8 to R.7.11 in Chapter R.7b and R.7c).These sections in Chapter R.7 also include information on how to derive appropriate information for classification and labelling of the substance. However, the rules on classification and labelling themselves are provided elsewhere. See current Annex VI to Directive 67/548 and future Guidance on Classification, Packaging and Labelling related to the future GHS system. Section 7.13 in Chapter 7c provides guidance related to the particular assessment approaches for hydrocarbons and metals.
- Overall framework for meeting the information requirements on intrinsic properties of substances (Chapter R.2), guidance on collection of available information (Chapter R.3), evaluation of information (Chapter R.4), guidance on exposure-driven waiving and exposure-triggered testing as well as other adaptations of information requirements (Chapter R.5), in depth guidance on non-testing approaches (Chapter R.6).
- Part B - Hazard Assessment (9/12/2011)
Part C contains the concise guidance on how to assess whether or not a substance is persistent, bioaccumulative and toxic (PBT) or very persistent and very bioaccumulative (vPvB). In-depth guidance on the PBT and vPvB assessment, including emission characterisation is covered in Chapter R.11.
- Part C - PBT Assessment (28/06/2017)
Part D - Framework for exposure assessment (17/08/2016)
Part D sets out the principles for carrying out an exposure assessment to determine the conditions of safe use for all the uses of a substance registered under REACH. It covers exposure for environment, workers and consumers.
Section D.2 describes the understanding that a registrant should have on the properties and the life cycle of his substances in order to determine his assessment approach and the corresponding scope of exposure assessment.
Section D.3 focuses in the characterisation of uses, providing principles to generate exposure scenarios (ES) and outlining key determinants playing a role in exposure levels for workers, consumers and the environment and provides advice on how to collect information on conditions of use.
Section D.4 provides an overview on how to derive exposure and release estimations.
Section D.5 briefly explains the risk characterisation that potentially triggers iterations of the initial exposure scenario. It also includes considerations on combined exposure and on handling uncertainty. More details on risk characterisation are provided in Part E of the guidance.
Section D.6 details the structure and requirements for preparing the chemical safety report, integrating in this guidance content from the obsoleted guidance Part F.
Section D.7 provides advice for developing the ES for communication to be annexed to the safety data sheet (SDS). Appendix D-1 list the headers for structuring the reporting of conditions of use within contributing scenarios (both for the chemical safety report (CSR) and for the ES for communication).
Part D provides also links to more in-depth guidance on exposure assessment, in particular how to describe uses, how to collect information on operational conditions and risk management measures, and how to carry out exposure estimates. This includes:
- Brief general description of identified uses and how to give exposure scenarios a short title (Chapter R.12)
- Risk management measures and operational conditions for building of exposure scenarios, including guidance on how to determine the effectiveness of risk management measures and how to make use of the risk management library initially set up during the development of the current guidance (Chapter R.13).
- Occupational exposure assessment (Chapter R.14)
- Exposure assessment related to consumers including releases from articles (Chapter R.15)
- Exposure assessment related to the environment including releases from waste life stage (Chapter R.16)
- Chapter R.18 provides guidance on exposure estimates related to waste life stage.
- Chapter R.20 explains the terms that are essential for the understanding of the Guidance.
Please note that the ES format guidance is now obsolete. Updated ES format can be found in the Practical examples of and Templates for exposure scenarios :
The ESCom package has been developed in the context of the CSR/ES Roadmap and provides an electronic format for exchange of ES information between IT systems.
The ECHA chemical safety assessment and report tool Chesar generates an ES for communication in line with all these initiatives above.
It is up to the individual registrant to decide which exposure scenario (ES) format he wants to use, as long as the content of the ES is compliant with the requirements set out in Annex I to REACH.
Part E contains the guidance on the risk characterisation. In risk characterisation, information on hazard and exposure is combined in the risk characterisation ratio or in qualitative risk characterisation. Both types of information contain uncertainty which needs to be assessed in order to decide on the robustness of the risk estimate. The uncertainty analysis is further detailed in Chapter R.19. Part E also includes guidance on qualitative risk characterisation with regard to non-threshold substances.
- Part E - Risk Characterisation (18/05/2016)
Please note that Part F is now obsolete. The content of the obsoleted Part F has been revised and merged into updated Part D (section D.6 and Appendix D-1).
Please note that Appendix to Part F is now obsolete. Updated CSR format can be found in the Illustrative chemical safety report and Templates for CSRs:
Please also note that ECHA's Chemical safety assessment and reporting tool Chesar and the report generator in IUCLID support the generation of the CSR.Please, note that Part G of the IR&CSA guidance has been made obsolete and hence removed from the ECHA website. The information has been updated and transferred to two more appropriate Guidance documents which have been recently revised. In particular:
- information on extending the safety data sheet and on how exposure scenario information is communicated and implemented by actors within the supply chain has been updated and included into Appendix 2 to the revised Guidance on the compilation of safety data sheets ;
- information on the use of scaling by the downstream user when evaluating whether he operates within the boundaries of the exposure scenario communicated to him has been updated and integrated into the revised Guidance for downstream users.
New practical examples on scaling are currently being developed to take into account the new experience gathered. These will be published when finalised. The old examples have therefore not been transferred to the revised guidance documents.
- Information requirements (Chapter R.2) (9/12/2011)
- Information gathering (Chapter R.3) (9/12/2011)
- Evaluation of available information (Chapter R.4) (9/12/2011)
- Adaptation of Information requirements (Chapter R.5) (9/12/2011)
- QSARs and grouping of chemicals (Chapter R.6)
- Appendix to Chapter R.6 for nanoforms (03/12/2019)
- Endpoint specific guidance (Chapter R.7a) (19/07/2017)
Note: this guidance is currently being updated. For the latest draft, please see consultation- Appendix to Chapter R.7a for nanomaterials (21/12/2022)
- Endpoint specific guidance (Chapter R.7b) (03/2024)
- Appendix to Chapter R.7b for nanomaterials (24/05/2017)
- Endpoint specific guidance (Chapter R.7c) (12/2023)
- Appendix to Chapter R.7c for nanomaterials (04/10/2021)
- Endpoint specific guidance (Chapter R.7.13-2)
- Characterisation of dose [concentration] - response for human health (Chapter R.8) (28/11/2012)
- Appendix to Chapter R8: Occupational exposure limits (01/08/2019)
- Appendix to Chapter R8: recommendations for nanomaterials (25/05/2012)
-
Please note that Chapter R.9 is now obsolete. The content of the obsoleted Chapter R.9 has been revised and merged into an updated sub-chapter R.7.1 "Physicochemical properties" within "Chapter R.7a: Endpoint specific guidance of the Guidance on information requirements and chemical safety assessment".
- Characterisation of dose [concentration] - response for environment (Chapter R.10)
- Appendix to Chapter R10: recommendations for nanomaterials (25/05/2012)
- PBT Assessment (Chapter R.11) (03/2024)
- Use description (Chapter R.12) (08/12/2015)
- Risk management measures and operational conditions (Chapter R.13) (24/10/2012)
- Occupational exposure assessment (Chapter R.14) (23/08/2016)
- Appendix to Chapter R14: recommendations for nanomaterials (25/05/2012)
- Consumer exposure assessment (Chapter R.15) (11/07/2016)
- Environmental exposure estimation (Chapter R.16) (16/02/2016)
- Please note that Chapter R.17 is now obsolete. The content of the obsoleted Chapter R.17 has been revised and merged into relevant sections of updates to Chapter R.15 (Consumer exposure assessment) and Chapter R.16 (Environmental exposure assessment).
- Estimation of exposure from waste life (Chapter R.18) (24/10/2012)
- Uncertainty analysis (Chapter R.19) (28/11/2012)
- Table of Terms (Chapter R.20) (18/09/2013)


