Q&As
Q&As
Q&A info
Doriți să căutați întrebarea și răspunsul relevant în limba dumneavoastră? Schimbați limba din meniul derulant de mai sus.
Înapoi
The Integrated Regulatory Strategy, developed in consultation with the Member State competent authorities, aims to encourage registrants to comply with REACH and to improve their chemical safety assessments.
The Integrated Regulatory Strategy has three main pillars: 1) efficient selection of substances which may raise potential concern; 2) generation of the necessary information through compliance check for assessing their safety; and 3) assurance that any remaining concerns are subsequently addressed through the most suitable regulatory risk management instrument.
It can therefore be assumed that the selected substances that raise potential concern will be more likely addressed with further regulatory risk management measures, and that the substances that demand most urgent action will be part of the prioritisation.
More information is available at the Integrated Regulatory Strategy -page.
- high tonnages;
- suspected data gaps in the higher-tier human health or environment information requirements;
- high potential for exposure of humans or environment (e.g. widespread uses or uncertain information on uses).
If your dossier meets the criteria for selection (see Q&A 647), ECHA will start a compliance check assessment, focusing on selected information requirements (or properties) (see Q&A 654).
When a compliance check for a substance is launched, ECHA informs concerned registrants via REACH-IT. This notification also reminds the registrants of their legal obligation to keep the registration dossier up-to-date (see Q&A 1572).
At the same time, the information on the dossier evaluation status page will be updated. From then on, you can follow the progress of the evaluation through the entire process (see Q&A 1572) or via the ‘ECHA Dossier Evaluation status’ section of the relevant joint submission page in REACH-IT.
If the information requirements (or properties) assessed are found to be non-compliant, the relevant members of the joint submission will receive a draft decision from ECHA.
No, by default ECHA assesses specific information requirements (or properties) (see Q&A 0654) primarily focusing on dossiers of substances manufactured or imported at more than 100 tonnes per year.
ECHA may also target its checks to different information requirements; then the decision will indicate the scope of the assessment undertaken.
REACH does not limit the number of compliance checks per dossier. Therefore you may receive multiple draft decisions on the same dossier.
The updated dossier containing the information requested needs to be submitted at the latest by the deadline set in the (adopted) decision (Article 22(2) of REACH). The updated registration dossier will be assessed after the deadline set in the decision has passed (Article 42 of REACH), on whether it contains the information requested in the decision.
This is however without prejudice to your legal obligation to update your dossier with any (new) relevant information “without undue delay” (Article 22(1)).
Under ECHA’s Integrated Regulatory Strategy (see Q&A 0646), the compliance check of dossiers submitted for substances manufactured or imported at more than 100 tonnes per year mainly focuses on eight key information requirements (or properties): genotoxicity, repeated dose toxicity, pre-natal developmental toxicity, reproductive toxicity, carcinogenicity, long-term aquatic toxicity, biodegradation and bioaccumulation.
The goal is to focus on the information requirements (or properties) that matter most for human health and the environment, with special emphasis on those which are related to the persistent, bioaccumulative and toxic (PBT) or carcinogenic, mutagenic or toxic to reproduction (CMR) properties of a substance.
More information on the strategy on compliance checks is available at compliance checks page.
ECHA informs active registrants via REACH-IT, when a compliance check for a substances in their portfolio is launched. This notification is a reminder of the legal obligation to keep the registration dossiers up-to-date, which is particularly important when a compliance check is initiated.
You can follow the progress of the evaluation via the ‘ECHA Dossier Evaluation status’ section of the relevant joint submission page in REACH-IT or through the Dossier Evaluation status webpage as explained below.
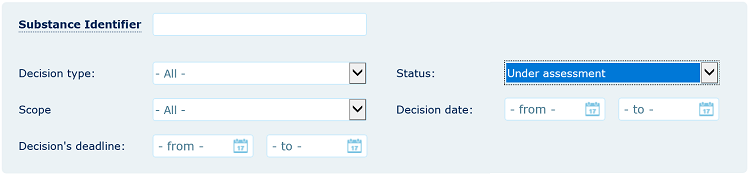
Please note that even if your substance appears under assessment, you may not necessarily receive a draft decision, since it is sent only to registrants that are concerned by the data gaps identified or since it is sent to a registrant for reasons specific to that registrant (a draft decision may be registrant specific in cases where the evaluation concerns substance identity information or endpoints from which a registrant has opted out).
Even if you do not receive a draft decision, you can continue to follow the progress of the substance through the Dossier Evaluation status webpage.
If you have received a draft decision, it means that there are non-compliant information requirements (or properties) in your dossier or in the jointly submitted registration dossier.
Check in the decision which members of your joint submission have received the draft decision, and get promptly in touch with them.
You have 30 days to provide your comments on the content of the decision. ECHA strongly recommends that you provide your comments collectively, in one consolidated set. ECHA only accepts comments submitted using the webform. A link to the webform is provided in the notification letter accompanying the draft decision.
For more information, see also the ‘Decision under dossier evaluation’ under recommendations to registrants and the practical guide ‘How to act in dossier evaluation’
- If the requested information already exists, you will need to agree on the costs and sharing of the existing data.
- If the requested information needs to be generated, you will need to:
- agree on the test to be performed, including on the testing material to be used as representative for all registrants;
- inform ECHA of who will perform the test; and
- share the costs and new data
For increased transparency on evaluation and other processes, ECHA has published supporting information on the following pages:
For more information, see also ‘Decision under dossier evaluation’ under the Recommendations to registrants and check the practical guide ‘How to act in dossier evaluation’.
As of January 2019, ECHA addresses its (draft) decisions to all registrants relying on non-compliant information requirements. Similarly, ECHA addresses its (draft) decisions on testing proposals to all those registrants intending to rely on the proposed tests to fulfil their information requirement.
Substance information exchange forums (SIEFs) ceased to exist as of 1 June 2018. Nevertheless, the registrants of the same substance continue to be bound by the obligation to submit jointly the information on their substance.
By setting out clearly how the information requirements apply at each tonnage band, ECHA’s decision gives the registrants greater certainty and clarity on their individual legal obligations, which helps ensure that all registrants within the joint submission become compliant.
Addressing evaluation decisions to all non-compliant registrants also aims to supports data and cost sharing as well as collaboration and communication between co-registrants regarding their joint submissions.
All recipients must comply with their information requirements, and will need to share existing/new data while respecting their legal obligation to avoid unnecessary (vertebrate animal) testing.
ECHA’s dossier evaluation draft decision is based on the version of the registration dossier available in ECHA’s database at the time the draft decision is issued to the registrants for comments. ECHA does not examine updates of your registration dossier that have been submitted after this process step. Therefore, make sure that information on your tonnage, uses and registration type are regularly updated to match the actual use of the substance, so that ECHA can assess its safe use in Europe.
Your legal obligation is to keep your dossier up to date – in other words, to update your dossier without undue delay if you become aware of new information. Therefore, the initiation of a compliance check should not be a trigger for updating your dossier.
When ECHA starts the evaluation of a dossier, information on the dossier evaluation status can be found at Dossier evaluation status page. Furthermore, when a compliance check is launched, ECHA informs concerned registrants via REACH-IT (see also Q&A 1572). Therefore, you have the opportunity to verify and (if necessary) update your dossier especially regarding use and tonnage information before a draft decision is issued.
In the specific case where you cease manufacture or import of your substance after receiving the draft decision, please see Q&A 1580.
In the specific case where you update your dossier with substantial new information, including tonnage band updates, after receiving the draft decision, please see Q&As 1927 and 1928.
For more information, see also ‘Dossier evaluation decisions’ under the Recommendations to registrants and check the practical guide ‘How to act in dossier evaluation’.
The REACH Regulation sets the deadlines which apply during the decision-making procedure. The deadlines will not be extended, unless there are technical reasons (e.g. malfunction of the submission tools) or the commenting period falls during closure periods of the Agency.
Similarly strict timelines are in place for the proposals for amendment by Member State competent authorities, the referral to the Member State Committee (MSC), your comments on the proposals for amendment, and the agreement-seeking on the draft decision by the MSC.
For more details, see the practical guide ‘How to act in dossier evaluation’.
If you decide to cease manufacturing or importing your registered substance upon receipt of the draft decision, you should inform ECHA of your intention using REACH-IT (see Q&A 1439). When you confirm that you have ceased/are ceasing manufacture or import, ECHA proceeds by invalidating your registration number in line with Article 50(3), after which you are not allowed to manufacture or import the substance into the EU/EEA market.
Consequently, you will not receive the adopted decision (if you have already received an adopted decision, see Q&A 1580).
If you start to manufacture or import the substance again, you will have to register the substance once more and you may have to share the costs accrued for the maintenance and update of the registration dossier due to an evaluation process or for other reasons.
For more information, see also the practical guide ‘How to act in dossier evaluation’ and ECHA’s Recommendations to registrants.
If after receipt of a draft decision you have updated your dossier with a lower tonnage band and you wish ECHA to consider the downgrade in the ongoing decision-making process, you need to inform ECHA in your comments on the draft decision, or if the commenting period has passed, in a communication to ECHA via the contact form. In that communication you should refer to the REACH-IT message which delivered the draft decision (in format CCH-D-XXXXXXXXXX-XX-XX/D). You also need to provide the annual volume of the substance that has been imported and/or manufactured over the preceding calendar year together with documentary evidence supporting this information. ECHA will take the elements available into account to determine whether the change of tonnage band has been substantiated and whether there is a need to amend the draft decision accordingly.
For information on downgrades after receipt of an adopted decision, please see Q&A 1580.
If the commenting period is over, but you have not yet received the adopted decision, you can inform ECHA of substantial new information that may have an impact on the decision by contacting ECHA via the contact form. In that communication you should refer to the REACH-IT message which delivered the draft decision (in format CCH-D-XXXXXXXXXX-XX-XX/D). In your communication you also need to reproduce the information contained in your update, explain why it was not submitted in your comments to the draft decision and explain how it affects the information requests in the draft decision. Substantial new information means:
- Recent experimental studies that became available to you after receipt of the draft decision and which address data-gaps identified in the draft decision.
- Tonnage band changes (for tonnage band downgrades the information referred to in Q&A 1927 needs to be provided).
- Registration type changes (you will need to comply with the conditions set out in Articles 17 and 18 of REACH).
No, ECHA cannot alter the deadline of the decision, because it is unanimously agreed by the representatives of the Member States.
ECHA acknowledges that technical difficulties in testing a substance may lead to delays or to the inability to provide the requested information by the deadline set in the decision.
In any case, we advise you to submit an update of your dossier by the deadline set in the decision, including any relevant explanations and evidence concerning the possible delay and the expected submission date. You should then update your dossier again as soon as the missing information is available.
However, if you believe that the technical difficulties prevent you from testing altogether, you can, on your own responsibility, adapt the standard information requirements (see Q&A 1064).
After the deadline, ECHA will check whether a dossier update is submitted and whether the information provided fulfils the information requirements. If during the follow-up evaluation ECHA finds that some or all of the requested information is missing, the national enforcement authorities will be informed.
No. ECHA considers that after 10 years of REACH implementation, there is broad knowledge in all industry sectors, and that they are well placed to support you.
However, if you have questions regarding a (draft) decision, you may submit your enquiry to ECHA using a contact form and we will assess your specific question.
No, ECHA cannot discuss the content of the adopted decision after it has been unanimously agreed by the Member States representatives.
If you have general questions on ECHA’s decisions or processes, you may submit a request to the ECHA Helpdesk and we will assess your concerns.
Yes. If you believe that you have scientific grounds to fulfil the requested information by adapting the requests in the decision, you may do so on your own responsibility.
Any adaptation must fulfil the specific rules outlined in Annexes VII to X or the general rules of adaptations specified in Annex XI to the REACH Regulation.
See also ECHA’s Recommendations to registrants.
Once the deadline set in the decision has passed and after ECHA has assessed whether the information you submitted is sufficient, ECHA will address the non-compliance through a new decision-making process according to Articles 50 and 51 of REACH.
This means that you will receive a new draft decision informing you that the information you submitted is not sufficient and that you still need to address the request in the original decision in order to fulfil the information requirements for the endpoint(s).
This decision has no deadline. Once the decision is adopted by the Member States, ECHA will inform the national enforcement authorities of the countries where the registrants are located.
If upon receipt of the adopted decision, you cease the manufacture or import of your substance, or if you downgrade the tonnage band of your registration, you will have to comply with the decision, regardless. See also Q&A 1586.
For more information, see also the practical guide ‘How to act in dossier evaluation’ and ECHA’s Recommendations to registrants.
See also Q&A 1574.
Check the end of the decision for the list of addressees of the decision and contact them. You will need to collectively agree on who will perform the test(s) (see Q&A 1583) and inform ECHA about this within 90 days of the adoption of the decision.
If no agreement is reached, ECHA will designate one of the registrants to perform the studies on behalf of all other addressees of the decision. For more information, see the practical guide ‘How to act in dossier evaluation’.
After you have collectively agreed on who will perform the test(s), you have to inform ECHA accordingly within 90 days from the receipt of the adopted decision (Article 53(1) of REACH).
Please use the link to the webform provided in the notification letter accompanying the adopted decision:
You must indicate a name for each request listed in the decision, even if it is the same for all requests. If you fail to do so, ECHA will have to designate one of the recipients of the decision, for each test lacking an identified registrant.
All recipients of the same decision are collectively responsible for fulfilling the requests in the decision that are relevant to their registered tonnage band.
After you receive the adopted decision, you need to agree collectively on who of the recipients is going to perform the requested studies. This is independent from the fact that the lead registrant is the one who submits the information “acting with the agreement of the other assenting registrants” (Article 11(1) of REACH).
The recipients of the decision are expected to agree on the testing to be performed as per their data (and cost) sharing obligations. You also need to agree on the material to be tested for each study requested, ensuring that it produces appropriate information and is representative of the registered substance as manufactured by all members of the joint submission.
You are also collectively responsible for the submission of the requested information by the (lead) registrant appointed to do so, on behalf of the other registrants of the joint submission.
If you do not update your registration by the deadline set in the decision, because you ceased manufacture, or import, or downgraded tonnage band after you received the adopted decision, ECHA will inform the Member State competent authorities and the national enforcement authorities of the country you are located in (see Q&A 1065).
Your authorities are responsible for any enforcement actions related to evaluation decisions according to your national legislation.
Any registrant receiving the adopted decision, because they are not compliant with their information obligations, remains legally responsible for providing the information requested in the decision (see Q&A 1583).
Consequently, a change of lead after you receive the adopted decision does not change the legal responsibility of the other recipients.
In addition, if ECHA identifies that the registrants have failed to submit and comply with the information requirements, it will notify the enforcement authorities of all the countries where the incompliant registrants are located. Any enforcement actions related to evaluation decisions will be decided according to their national legislation, where appropriate.
No. ECHA will only check whether a dossier update is submitted once the deadline set in the decision has passed. ECHA will then evaluate whether the submitted information (test or adaptation) fulfils the requests in the decision (see Q&A 1064). This is part of the follow-up evaluation process.
In other words, ECHA does not perform any evaluations before the deadline in the decision has expired.
Once the deadline set in the decision has passed ECHA will assess the dossier. In case the information requested in the decision are not submitted, ECHA will inform the Member State competent authorities and the national enforcement authorities of the respective Member State about the failure to respond to the request(s) in the decision.
When ECHA sends information to national enforcement authorities, the responsibility for handling the non-compliance with REACH is transferred to the national authorities, which then consider and decide on enforcement actions, where appropriate.
Consequently, ECHA will not evaluate any subsequent dossier updates unless the respective national authority asks ECHA to do so.
You should direct all questions to your national authorities.
ECHA appreciates that the current global emergency has affected several registrants in the EU. For this reason, ECHA extended certain deadlines in the registration and evaluation processes, for a limited period of time, to take account of the current global situation (see more here).
However, the Agency is not in a position to alter the final deadlines set in adopted decisions. This is because these are decisions that have been agreed in close consultation with the Member States.
As usual, ECHA will initiate the follow-up evaluation of the updated registration dossier for the substance subject to dossier evaluation process that you mentioned in your document when the deadline in the decision has passed. Then, ECHA will establish whether the new information submitted in an updated dossier corresponds to the requests in the decision.
If you think you will not be able to provide all the information requested in the decision by the deadline set, we advise you to anyway update your registration dossier by the deadline and, if necessary, include all relevant explanations and proof concerning the status of ongoing tests and the reasons for the delay, including the expected submission date. As soon as the missing information becomes available to you, you should update your registration dossier again.
According to Article 42(1), ECHA needs to examine all of the information submitted as a response to a testing proposal and compliance check decision. The Agency also has to draft any appropriate decisions, if necessary.
If the information provided in the dossier update does not fulfil the information requirements, the Member State competent authorities will be informed. They will then have to consider your argumentation and decide on any enforcement actions, where appropriate.
For further information on the follow-up to dossier evaluation decisions, please see the steps in the Evaluation process and the answers to the most frequently asked questions on Evaluation on the ECHA website.


