Q&As
Q&As
Q&A info
Haluatko etsimäsi kysymyksen ja vastauksen omalla kielelläsi? Vaihda kieltä yllä olevasta vetovalikosta.
Takaisin
REACH-IT has been tested with:
- Google Chrome 48.0 and higher on a Microsoft Windows platform
- Internet Explorer 11.0 and higher on a Microsoft Windows platform
- Mozilla Firefox 44.0 and higher on a Microsoft Windows platform
The use of untested browsers may reduce REACH-IT functionalities and cause application errors in REACH-IT. Using an older version of the browsers, or different software platform, may cause incompatibility issues with REACH-IT functionalities. Therefore, users are advised to upgrade their internet browser as mentioned above.
There is no need to synchronise the company details in REACH-IT and IUCLID. The legal entity information is always extracted from the information in REACH-IT.
However, if you wish to do so, we recommend updating the information in REACH-IT, and later exporting it to your IUCLID 6. If you update your LEO information in your IUCLID 6 application, remember also to update your LEO information in REACH-IT.
For more information, see the manual How to prepare registration and PPORD dossiers, chapter 2.1, How to update and synchronise the LEO information.
REACH-IT and IUCLID support the following file types: jpg, jpeg, pdf, doc, docx, rtf, txt, tiff, mol, png, gif, xls and xlsx.
The EC number can be downloaded from REACH-IT either from the Joint submission Object, the Inquiry or the Pre-SIEF page.
- Log in to REACH-IT, go to the relevant submission page and click on the button ‘Export assigned EC number or Export EC substance’, as shown below:

- Once the assigned EC number has been exported, go back to the IUCLID home page and click on ‘Import’ as shown below.

- Once the assigned EC number has been imported go back to the IUCLID home page and click on ‘Reference substance’ as shown below.

- Click on the button ‘New reference substance’ as shown below.

- ‘Add’ the newly uploaded reference number to the reference number inventory by putting the name of the reference substance and click create and open.

- Fill in the reference substance identity information and save the entry.
After you have submitted your registration dossier to ECHA you may realise that you made a mistake during its preparation. This might be the case, for example, if you accidentally introduced faulty information in the dossier (e.g. incorrect information in one of the study summaries) and noticed this only after you submitted the dossier to ECHA. In this case you should without undue delay submit the amended dossier as a spontaneous dossier update via REACH-IT, indicating in the dossier header the reason(s) why you are spontaneously updating it as well as the references of the previous valid submission (i.e. the "last submission number"). Such an update would not be subject to a fee, unless the mistake is related to a chargeable element such as an increase in tonnage band, or confidentiality claims for information listed in Article 119(2).
Some mistakes may prevent a successful submission of the dossier to ECHA. These are failures in the business rules or the completeness check. If your submission fails either of these checks, you will be alerted to the failure in the form of a task in REACH-IT, which prompts you to amend the dossier.
Following the entry into force of the ‘Commission Implementing Regulation (EU) 2016/9 on Joint Submission of Data and Data Sharing', new checks were put in place to ensure that all submissions are in line with the ‘one substance, one registration' (OSOR) principle. According to the OSOR principle there can only be one joint registration per substance per registration type (full or intermediate).
Furthermore, if an active joint submission already exists (i.e. the lead registrant has successfully submitted the lead dossier), individual registrants (i.e. not part of the joint submission) for the same registration type (full or intermediate) are blocked from updating their dossiers, unless they become members of the joint submission.
ECHA advises you to take the following steps:
- Find your co-registrants.
- Establish substance sameness and negotiate on data sharing.
- For the scenarios described in Q&A 1171, you can partially or fully opt-out from information submitted jointly by your co-registrants. However, your submission still has to be in the framework of the joint submission. Therefore, certain administrative fees may be applicable towards the joint submission.
- If you cannot agree on the data and cost sharing, you can file a data-sharing dispute with ECHA. The dispute mechanism should be used as a last resort and you will need to demonstrate that you have made every effort to reach a voluntary agreement.
- If you wish to submit a partial or full opt-out, but cannot come to a voluntary agreement with the lead registrant on the conditions for joining the joint submission, you can also use the above-mentioned dispute mechanism.

Business rule 027 (BR027) failure occurs if the substance identifier in your IUCLID dossier does not match the substance identifier on the REACH-IT joint submission page. To avoid BR027 failure in your future submissions, see the following check list and carry out the steps that apply to your case.
- Check that you have selected the correct joint submission in REACH-IT when submitting your IUCLID dossier.
- Check that the EC number in your IUCLID dossier is the same as the EC number of the joint submission in REACH-IT. You can export the assigned EC number either from the REACH-IT joint submission page, from the Pre-SIEF page, or from the inquiry submission report page. Import the EC number into IUCLID and assign it to your reference substance, as specified in Q&A 1258.
- If no EC number exists for your substance yet, check that the spelling of your substance name on the REACH-IT joint submission page is identical to the spelling in your IUCLID dossier section 1.1 IUPAC name. Pay attention to additional spaces and special characters.
- If your substance is a multi-constituent substance (e.g. a reaction mass of…), make sure that all the constituents indicated in section 1.2 of IUCLID are identical to the constituents included in the REACH-IT joint submission page.
- If you are a lead registrant of a new joint submission for a substance of Unknown or Variable composition, Complex reaction products or Biological origin (UVCB) with no valid pre-registration or inquiry number, and have tried the above mentioned fixes, please contact ECHA using the ECHA contact form to receive further instructions on how to successfully submit a registration.
While creating your dossier, ECHA advises you to use the IUCLID Validation assistant plug-in to help you to detect any business rules and technical completeness check failures in your dataset and dossier, as explained in Q&A 0392. Please address any detected failures before re-submitting your dossier update to ECHA through REACH-IT.

Business rule 034 (BR034) failure occurs when you submit a spontaneous update instead of requested update. When your previous submission fails the technical completeness check (TCC), you are expected to submit a requested update. A communication regarding the TCC failure is sent to you via REACH-IT. You also receive an update request under ‘Tasks’ in your REACH-IT account with instructions on how correct the TCC and submit a requested update.
When you create your dossier update, you must tick the boxes 'The submission is an update' and 'Further to a request/decision from a regulatory body’.
Add your last submission number, in this case, the submission that failed the technical completeness check. In the box below, enter the communication number in the ‘Number’ field, as shown in the screenshot.

You can find the submission number and the communication number in REACH-IT under ‘Tasks’ and also under ‘Key documents’.
While creating your dossier, we also advise you to use the IUCLID Validation assistant plug-in to help you to detect business rules and technical completeness check failures in your dataset and dossier, as explained in Q&A 0392. Please address any detected failures before re-submitting your dossier update to ECHA through REACH-IT.

Business rule 35 (BR035) failure occurred because the last submission number you have indicated in your dossier is incorrect. To address the failure, you will need to indicate the last successful submission number for the particular substance in the dossier header in IUCLID, and then re-submit the dossier in REACH-IT.
You can find your previous successful submission number in REACH-IT by going to ‘Substances’ and searching for your registration using the substance name or EC number. The submission number is presented in the results page, as shown in the screenshot below (1).
If you are expected to submit an update due to technical completeness check failure, you need to use the ‘In progress’ submission number (2).
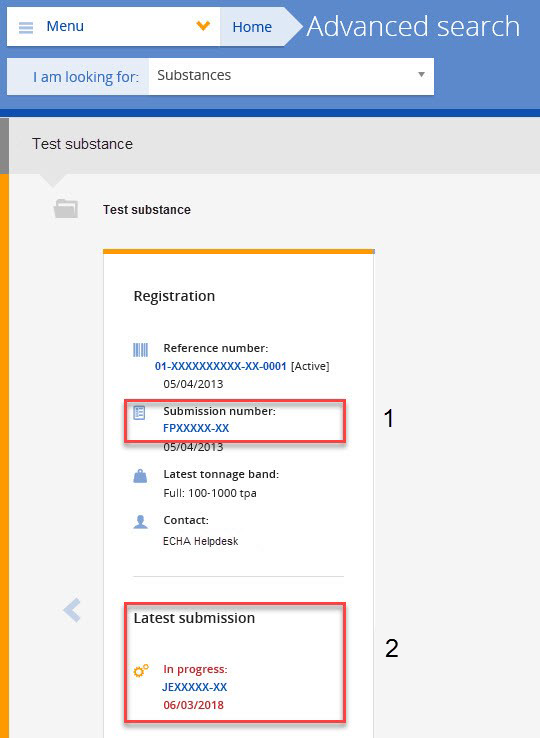
Assign the correct submission number in the IUCLID dossier creation wizard by ticking the box ‘The submission is an update’ and insert the last submission number, as shown in the screenshot.
While creating your dossier, we also advise you to use the IUCLID Validation assistant plug-in to help you to detect business rules and technical completeness check failures in your dataset and dossier, as explained in Q&A 0392. Please address any detected failures before re-submitting your dossier update to ECHA through REACH-IT.

Your submission has failed business rule 038 (BR038) because you already have a successful submission for the substance and you are expected to submit an update.
To address the failure, you need to assign the last successful submission number of the substance you are submitting the update for in the IUCLID dossier header. Indicate the reason for the update by choosing the appropriate update type, ‘Spontaneous update’ or ‘Further to a request/decision from a regulatory body’.
You can find your last successful submission number in REACH-IT by going to ‘Substances’ and searching for your registration using the substance name or EC number. The last successful submission number is listed on the results page, as shown in the screenshot below.

For a spontaneous update, carry out the following steps, as shown in the screenshot below (Desktop view and IUCLID Cloud view).
- Assign the submission number in IUCLID dossier creation wizard by ticking the box ‘The submission is an update’ and inserting the last successful submission number that corresponds to the substance.
- Select the type of update, ‘Spontaneous update’.
- Select the appropriate justification for the update. Create a block by clicking the button "New item" and make a selection from the drop down list. If you select ‘other’, you are required to give the reason in the adjacent free text field.

For a requested update, refer to the assessment outcome communication sent to you via REACH-IT. You can find the communication on the submission page under the ‘Key documents’ tab. The communication provides full details on what information is missing from the dossier and a deadline by when you should submit this information.
For a requested update, carry out the following steps, as shown in the screenshot below (Desktop view and IUCLID Cloud view).
- Assign the latest submission number in IUCLID dossier creation wizard by ticking the box ‘The submission is an update’ and inserting the last successful submission number, which corresponds to this substance.
- Select the type of update, ‘Further to a request/decision from a regulatory body’.
- Create a block by clicking the button "New item" and insert the annotation number that corresponds to the submission for which further information was requested (e.g. assessment outcome communication number XXX-X-0000000000-00-00/X). In the ‘Remarks’ field, enter your remarks as free text.

While creating your dossier, ECHA advises you to use the IUCLID Validation assistant plug-in to help you to detect any business rules and technical completeness check failures in your dataset and dossier, as explained in Q&A 0392. Please address any detected failures before re-submitting your dossier update to ECHA through REACH-IT.

Your submission has failed business rule 053 (BR053) because the EC/list number in section ‘1.1 Identification’ your IUCLID dossier does not match the EC/list number of the inquiry indicated in section ‘1.3 Identifiers’.
To address the failure, you need to ensure that:
- the substance identification in section ‘1.1 Identification’ of the IUCLID dossier corresponds to the substance identification associated to the inquiry number indicated in section ‘1.3 Identifiers’;
- the inquiry number indicated in section ‘1.3 Identifiers’ has been copied/typed in the correct format.
To select the EC/list number provided by ECHA as a result of your inquiry assessment, you need to export this EC number from REACH-IT in .i6z format into IUCLID. For instructions on how to export the EC number from REACH-IT to IUCLID, see Q&A 1258.
You can find your inquiry number in REACH-IT by going to ‘Substances’ and searching for your inquiry using the substance name or EC number. The inquiry number (e.g. 06-0000000000-00-0000) will be listed on the results page.
While creating your dossier, ECHA advises you to use the IUCLID Validation assistant plug-in to help you to detect any business rules and technical completeness check failures in your dataset and dossier, as explained in Q&A 0392. Please address any detected failures before re-submitting your registration dossier to ECHA via REACH-IT.

Business rule 119 (BR119) failure occurs when the lead registrant indicates in their dossier update that they will not provide the optional information (i.e. chemical safety report (CSR) and guidance on safe use (GoSU)) on behalf of its members although in the previous submission they had indicated that they would provide this information.
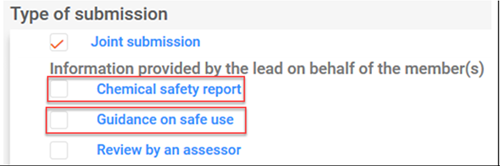
The following scenarios may trigger BR119 failure:

If the lead registrant initially indicated that they would provide the CSR and/or GoSU on behalf of all members of the joint submission but no longer wishes to do so, they should contact ECHA using the ECHA contact form and request the modification of the joint submission coverage. Before contacting ECHA:
- The lead registrant should first ensure that the changes will not affect any members of the joint submission.
- Members relying on the CSR and/or GoSU provided by the lead registrant need to submit a spontaneous update indicating that they will provide individual CSRs and/or GoSUs.
Once ECHA has performed the changes, the lead registrant will need to submit a spontaneous update.
When submitting the spontaneous update, member or lead registrants should indicate in the IUCLID dossier the submission type as ‘spontaneous update’, the ‘Justification’ select the reason ‘New or update of CSR or guidance of safe use’ as well as entering a clarification in the ‘Remarks’ text box.
While creating the dossier, we advise you to use the IUCLID Validation assistant plug-in to help you to detect business rules and technical completeness check failures in your dataset and dossier. For further instructions on how to use the validation assistant please refer to Q&A 392.

Business rule 130 (BR130) failure occurs when a lead registrant submits a dossier update in which the joint submission tonnage band indicated is lower than the joint submission tonnage band for the previous submission. The tonnage band of the joint submission is derived from the submission type (IUCLID template) chosen for the substance when the lead dossier was first created.

The individual tonnage band(s) specific to the lead registrant can be indicated in the dossier header.

If the lead registrant wishes to lower their individual tonnage band, they can refer to Q&A 1300 for further instructions.
If the lead registrant wishes to lower the tonnage band of their joint submission, they can refer to the Q&A 1299 for further instructions.
While creating your dossier, ECHA advises you to use the IUCLID Validation assistant plug-in to help you to detect any business rules and technical completeness check failures in your dataset and dossier, as explained in Q&A 0392. Please address any detected failures before re-submitting your registration dossier to ECHA via REACH-IT.
- Save the downloaded file locally on your computer.
- Open Excel. In the “Data” tab click on "Get Data -> From File From Text/CSV" to open a wizard.
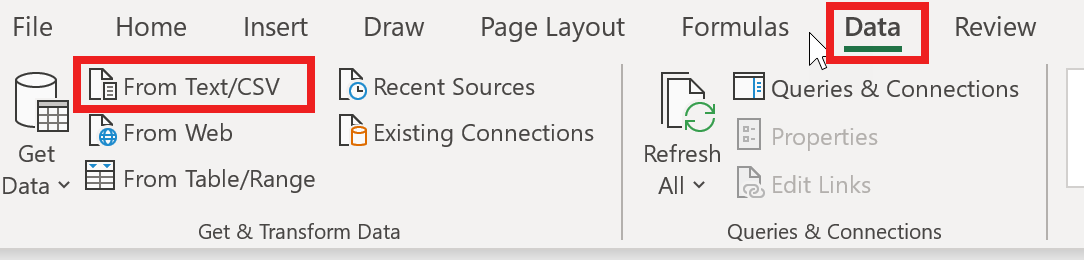
- Go to the location of the CSV file and import it.
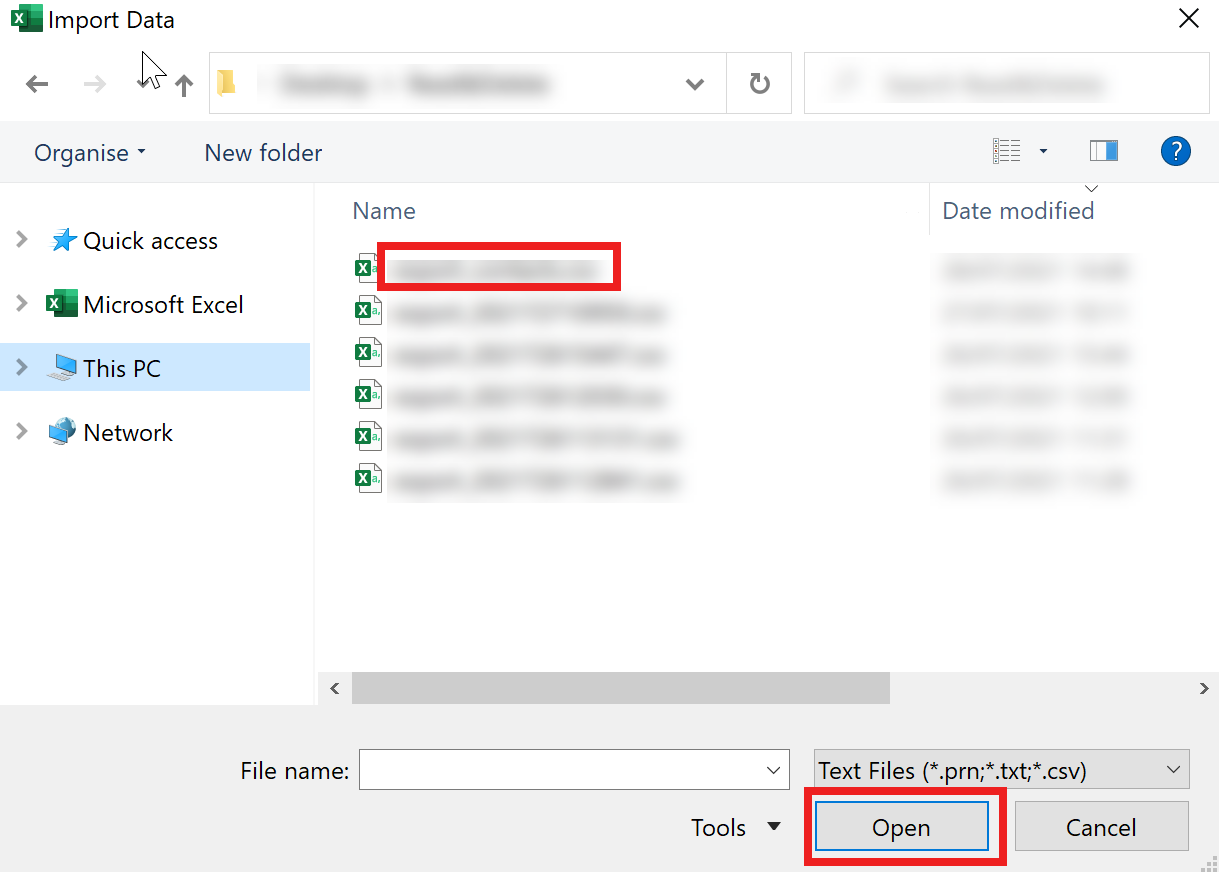
- The wizard will open. Set the “File Origin” (character encoding) to “65001: Unicode (UTF-8)” from the dropdown list.

- Set the “Delimiter” type to “Semicolon” from the dropdown list.

- Verify that the example data are correct and select “Load”.

- In case you need to make any changes, select the “Transform Data” option.


