Q&As
Q&As
Q&A info
Soovid otsida asjakohaseid küsimusi ja vastuseid oma keeles? Muuda keel ülal rippmenüüs.
Tagasi
- The list of biocidal active substances for which an application for approval has been submitted, approval has been granted or refused. The published information includes also documents such as the assessment report, study summaries (Document IIIA), opinion and a link to the related legal text approving or not approving substance to be used as a biocidal active substance.
- The list of union, simplified and national biocidal product authorisations. The published information includes also documents such as the assessment report, summary of product characteristics, decision or authorisation document, and in case of union authorisations also the Opinion and link to the related legal text.
- The list of active substances and suppliers (“Article 95 list”).
The biocidal product factsheet contains detailed information on the biocidal product’s authorisation as available in R4BP 3. The factsheet also includes a map with the authorisation status of a product, authorisation details of all mutually recognised applications and historical data on previous assessments.
A biocidal product factsheet also contains the:
- Summary of product characteristics (finalised or updated after 1 January 2016 in R4BP 3).
- Product assessment report.
- Product authorisation/decision documents.
- Biocidal product committee opinion (for Union authorisations).
The biocides data available in the “Information on chemicals” section on the ECHA’s website originates from the information provided by applicants and verified by the evaluating competent authority. ECHA does not verify the adequacy or the correctness of information provided that falls under the responsibility of the national authorities. For confidential data assessed by your evaluating competent authority, you will need to ensure the adequacy of the information published with your national authorities.
For further information on this topic, see Q&A 1554 and Q&A 1555.
Information on biocidal product authorisations will be published in the “Information on chemicals” section on ECHA’s website when a product asset is created in R4BP 3. Information on active substances will be published when the evaluation is started by the evaluating Member State authority.
- The summary of product characteristics (SPC) in XML format. Note: information on the function of other substances or of substances of concern will not be made publicly available.
- Documents under the “Documents” tab with access level ‘Public’:
- Active substance / product assessment report
- Product authorisation/decision documents
- Active substance Document III-A
- Active substance / Union authorisation Opinion
When you notice that certain published information from your active substance/authorisation is inaccurate, incorrect or missing, please contact the Member State responsible for evaluating your application. ECHA will publish the updated information as soon as the changes are applied in R4BP 3.
For other reasons, contact ECHA.
The most common reasons are:
- Data from active substance or biocidal product applications must be available in R4BP 3, i.e., new applications or updates must be authorised by your authorities in R4BP 3.
- For biocidal products, the information is derived from assets available in R4BP 3. Information from pending cases is not published.
The extended search functionalities allows you to search for products based on trade names, uses of the product (target organisms, application method, etc.) or authorisation type. You can also compare different biocidal products based on the data in the summary of product characteristics, such as the active substances in the product, their concentrations, product use information, and hazard and precautionary statements.
When more than one SPC is provided in the same biocidal product authorisation, i.e., different language versions, the “Information on chemicals” website will aggregate this information and provide – as search results – any possible different information available in different language versions. If certain information from the SPC is missing or invalid in a simplified authorisation or national authorisation, you may contact your national authorities to modify the information available in these SPCs.
The search results available in the “Search for chemicals” entry available in the ECHA homepage displays results derived from notifications, registrations or applications submitted from different regulatory context such as REACH, CLP, BPR or PIC. ECHA displays information on chemicals following an aggregation process that considers the substance identification, as provided by data submitters, in different regulatory context.
In order to ensure that the information on a specific active substance relates only to the BPR, click on the Biocidal active substances to ensure that the substance identity and related data refers solely to the BPR.

Alternatively, you may search directly using the Biocidal active substances section of the “Information on chemicals” portal.

The following options can be found under the “Approval/Assessment status” of a specific active substance and product-type combination:
FINAL DECISIONS
- “Approved”: positive decision on approval adopted by the European Commission.
- “Not approved”: decision not to approve adopted by the European Commission.
- “No longer supported”: the Commission issues a non-approval decision of an active substance/PT initially included in the review programme.
- “Cancelled”: application where the assessment of the evaluating Competent Authority is not continued any longer.
- “Expired”: the active substance/PT is no longer valid, nor an application for renewal has been submitted.
APPLICATIONS IN PROGRESS
- “Initial application for approval in progress”: depending on the status of the application, the application can be pending the evaluation of the appointed competent authority, the opinion of the Biocidal Product Committee (BPC) or the Commission decision.
Other scenarios may apply, such as an invitation to take over the role of participant in the review programme or a decision made by participants in the review programme not to support an active substance/PT pending the Commission decision. - “Renewal in progress”: while the initial application for approval has already been completed with a positive decision by the European Commission, a renewal application to extend the validity period of the relevant active substance/PT combination is in progress.
- “Other updates in progress”: the Commission reviews the conditions for approval of an active substance/PT combination according to Art. 15 of the BPR or there is a need to further submit additional data in accordance with the BPC opinion.
Additional information on the main steps can be found in the existing active substance process and new active substance process.
The Classification & Labelling (C&L) Inventory is a database which contains classification and labelling information on substances notified under Regulation (EC) No 1272/2008 (the CLP Regulation) and registered under Regulation (EC) No 1907/2006 (the REACH Regulation). It also contains the list of legally binding harmonised classifications (Tables 3.1 and 3.2 of Annex VI to the CLP Regulation). It is established and maintained by ECHA.
The C&L Inventory serves multiple purposes:
- It is a tool for hazard communication and a source of basic information on substances placed on the market which meet the criteria for classification as hazardous or are subject to registration, for suppliers of substances, the general public and Member State Competent Authorities (MSCAs);
- It reveals differences in the classification and labelling of the same substance applied by different suppliers, thus pointing to the need for further discussion among companies to explore the reasons for differences and/or agree on the correct classification, evaluation needs or the need for a legally binding harmonisation of a particular classification and labelling of a substance;
- It is an important tool for hazard communication and risk management, e.g. when MSCAs assess the need for potential authorisations and restrictions of hazardous substances under REACH.
The Classification & Labelling (C&L) Inventory is a database which contains classification and labelling information on substances notified under Regulation (EC) No 1272/2008 (the CLP Regulation) and registered under Regulation (EC) No 1907/2006 (the REACH Regulation). It also contains the list of legally binding harmonised classifications (Tables 3.1 and 3.2 of Annex VI to the CLP Regulation). It is established and maintained by ECHA.
Article 42 of the CLP Regulation and Article 119(1) of the REACH Regulation stipulate which elements of a notification should be publicly accessible in the Classification and Labelling Inventory. These consist of certain elements of the substance identity and all classification and labelling (C&L) elements. The EC name and number of all notifications for EINECS substances and, wherever possible, all other substances in the EC inventory, are published. In addition, when a substance is classified in certain hazard classes referred to in Article 119(1)(a) of the REACH Regulation by at least one notifier then the C&L elements are published from all notifications for that substance. The IUPAC name is only published from notifications classifying in the hazard classes referred to in Article 119(1), however.
The Public C&L Inventory does not contain contact details of notifiers or registrants. In order not to disclose confidential business information, no detailed information on impurities or additives is included in the Public C&L Inventory either. In addition, notifiers and registrants have the possibility to claim the IUPAC name confidential (further information on how to flag the IUPAC name confidential can be found in the manual Dissemination and Confidentiality under REACH Regulation). If so, it will not be included in the Public C&L Inventory.
The information published in the C&L Inventory is not reviewed or verified by ECHA or any other authority and can be changed without prior notice. ECHA does not guarantee the correctness of the information published in the database as it is automatically disseminated from the notifications and registration dossiers.
The notifications for each substance are grouped together based on numerical identifiers such as EC or CAS numbers, where they exist. The aggregation is done automatically based on both classification and labelling elements.
- Identical classifications are aggregated and displayed as one entry. The Number of Notifiers behind each aggregated classification is indicated.
- Different States/Forms of the substance (e.g. liquid, solid:bulk, solid:particulate/powder, solid:nanoform) are added under Additional Notified Information.
- Classifications derived from joint submissions to the REACH registration process are marked ‘yes’ in the Joint Entries column.
- Reasons for no classification are not taken into account during aggregation. They are reflected in the View details section of each classification entry.

One of the objectives of the C&L inventory is to promote uniform classification of substances. Notifiers have the legal obligation to make every effort to come to an agreed entry to be included in the inventory and inform ECHA accordingly (see Article 41 of the CLP Regulation).
For many substances different classifications have been notified to ECHA. There are several reasons for the range of different classifications:
- slight differences in seemingly identical notifications (e.g. differences in affected organs or route of exposure);
- different interpretation of scientific studies or different access to such studies;
- technical errors made during the notification process (e.g. not assigning all labelling elements correctly)
There may be other legitimate reasons for different classifications for the same substance:
- different compositions or impurity profiles often lead to different classifications;
- physical state and form of a substance is often very important when the hazards of a substance are assessed. The Public C&L Inventory displays the notified state and form but does not contain any information on composition or impurities.
The C&L Inventory displays notified classifications as they are reported to ECHA without any verification by the Agency. The notifications reflect, therefore, the current situation on the market. While notifiers have an obligation to undertake all efforts to come to an agreement on the classification for their substance, many may legitimately differ based on e.g., impurities or composition. We encourage all users to discuss their concerns with their suppliers but there is no single "correct" classification identified and highlighted by ECHA.
The C&L Inventory indicates where a legally binding harmonised classification is included in Annex VI of the CLP Regulation and where the classification of a substance was derived from the joint submissions to the REACH registration process.
Ceasing of manufacture or import does not mean that a substance is no longer present on the market. For this reason, the classification and labelling information of a substance remain available in the C&L inventory even after the manufacture or import has been discontinued.
When notifiers wish to notify a substance for which they believe no classification is required, they can tick the appropriate tick-box (labelled "not classified").
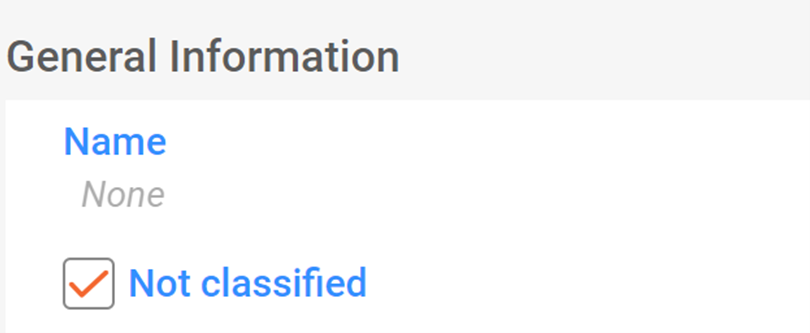
In this case, no further details on the classification and labelling of the substance are needed. The C&L Inventory displays these notifications with the label "not classified" and the third page view is disabled.
Some notifiers have submitted notifications with no C&L elements without ticking the “Not Classified” tick-box. For these notifications, ECHA cannot verify whether their intention was to submit no classification or whether the notifier simply forgot to add the C&L elements. These notifications are therefore displayed separately.
At the moment, it is only possible to download part of the C&L inventory in batches using the advanced search (accessible from the home page). The current search functionalities of the advanced search are based on classification only. Therefore, notified substances which are not classified as hazardous won’t be returned by the current searches.
If you want to download part of the C&L inventory, use the starting digits of the EC numbers to limit the number of entries in a batch. We suggest using the following: 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 3, 4, 5, 6, 7 ,8, 9.

A substance can be identified by searching either with the CAS number or the official EC number. For a substance with a harmonised classification and labelling, the Annex VI Index number, or the substance name can be used.
Whilst there is an information filter option present on the C&L Inventory portal page, the Advanced Search function present on the ECHA homepage is a far more powerful and versatile tool for finding relevant information in regard to Classification and Labelling, allowing for selection of specific hazard classes and selection of the information source.
These options can be combined with any of the other search functions of the Advanced Search, including most obviously, the substance identifiers section.
ECHA has noticed that in individual cases a wrong substance name has been supplied by notifiers with their notification. As ECHA displays the information as provided in the notifications, without verification of the accurateness of the data, this may result in spurious results when searching by substance name, as a seemingly unrelated substance could be displayed in the results. In such cases, it is advised to use the second page view where all published IUPAC names are listed, to identify whether the initially displayed name was incorrect. The grouping of substances is based on numerical identifiers and is not affected by inaccuracies in the substance name.

Yes, it is possible to download and export the search results in excel or csv format. No classification and labelling information can be downloaded.
Reproduction or further distribution of search results may be subject to copyright protection. Please note that using this information without obtaining the permission from the owners of the respective information might violate the rights of the owner. ECHA is not responsible for any copyright or other infringements that may be caused by you using the information.
No, all harmonised DSD classifications have been erased from public view as these are no longer relevant.
Substances with a harmonised classification and labelling (CLH) are listed in Annex VI to CLP. You can also find if the classification is harmonized by examining the text below the pictograms in the relevant substance InfoCard.

Alternatively, you can look for your substance in the C&L inventory and open the summary of classification and labelling page.
You should look for your substance in the C&L inventory and open the summary of classification and labelling page to find if your substance is covered by the Seveso directive.
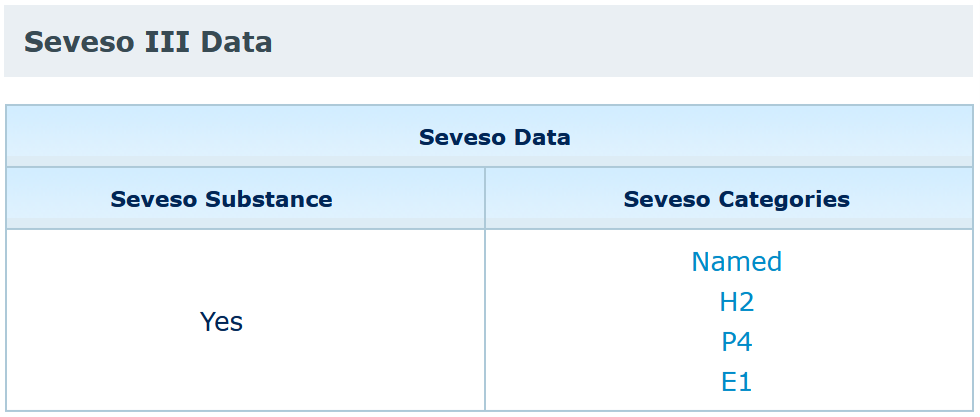
Please note that ECHA is not an authority for the Seveso Directive and that the Seveso categorisation is provided for information only. The Seveso III Directive (Directive 2012/18/EU repealing Directive 96/82/EC (Seveso II) from 1 June 2015) is the only authentic legal reference and that the information in this inventory does not constitute legal advice. For further information on Seveso, please ask your national authority.
- Validation assistant, which enables you to validate your registration dossier before submitting it, i.e. it performs a Business Rule check to ensure that you included a justification along your confidentiality claim;
- Dissemination Preview tool, which simulates which information from your dossier will be published on the ECHA website;
- Fee Calculator tool, which calculates the costs of your confidentiality claims and any other fees associated to your registration.
Indeed, we send the decision (acceptance/rejection) of a confidentiality claim via REACH-IT at the end of the assessment procedure.
If the justification is not sufficient, you will receive a communication detailing which information needs to be updated in order for your claim to be accepted. During the assessment of the confidentiality claim, the information claimed confidential is not published on the ECHA website.
After the assessment is concluded, the information found to be validly claimed confidential will not be made public. However, in case of rejected confidentiality claims, the information will be published on ECHA’s website.
For further information on the assessment procedure, see the Dissemination and Confidentiality under the REACH Regulation manual, section 3.8.
Please see our explanatory document on How to protect you confidential business information, section 1.
ECHA combines data per substance from all regulatory activities, therefore the information may be made publicly available on the ECHA website if not claimed to be kept confidential in all submissions. I.e. if another company submits e.g. a C&L notification or a registration for the same substance without claiming confidentiality on the substance name, it is published on the ECHA website.
Also, the validity of a confidentiality claim may be limited in time. If the validity of a confidentiality claim has expired in any of the submissions, the corresponding information is published on the ECHA website.
If you are not sure why the information is published, you can contact us.
Each item falling under REACH Article 119(2) must be separately claimed in the dossier and the reason for confidentiality must be justified also separately for each of them. The validity of these confidentiality claims is assessed.
The justification for each confidentiality claim needs to include: (1) a declaration that the information is not in the public domain or general knowledge in the industry, (2) a demonstration of a commercial interest worthy of protection for non-disclosure of the information, and (3) a demonstration that disclosure of the information would cause potential harm to the commercial interest of the registrant or a third party.
To claim confidentiality and insert the justification template in IUCLID web interface, open the flag next to the item you wish to keep confidential and select the confidentiality flag; CBI, IP or no PA. The template for justifying a confidentiality claim can be directly inserted in the justification field by selecting ‘Insert existing templates’ on the top right corner of the justification text box. After inserting the template, you can start editing it.
For dossier updates, justifications previously attached to the dossier in pdf format can be maintained.
- Ensure that the information claimed confidential is not already publicly available elsewhere.
- Add the CBI flag next to the information you wish to claim confidential and provide a valid justification.
- Run the Dissemination preview tool both in your IUCLID dataset and created dossier, to see which information from your registration dossier will be removed before publication and which information will be publicly available.
- Run the Fee calculator to see the applicable fees you need to pay.
Please contact us via our contact form, as soon as you notice that the information has not been flagged as confidential.
In the meantime, your latest successfully submitted dossier will be published and only the flagged confidential information will be removed from publication, pending confidentiality assessment.
You will be informed of the confidentiality assessment outcome via REACH-IT. In case of acceptance, the information you wish to not make publicly available will remain confidential.
In case of rejection, you will be asked further information before the confidentiality assessment is finalised and before the information is published.
Under REACH, only information entered into a IUCLID field is published. Attachments are not published.
- Click on “Menu” in REACH-IT
- Select “inquiry” as the type of dossier upload
- Upload the IUCLID dossier in the dossier submission wizard
- Continue to “Additional details”
- Click “Assign” next to “contact person”
- Click “Assign” next to the “Third party representative” field where you can assign an existing TPR or create a new one
- Continue to the “Confirmation” step of the wizard
Tasks for lead registrants
As a lead registrant, you can assign a TPR for the joint submission before submitting your registration dossier. Follow the below steps to assign a TPR for the joint submission:
- Click on “Menu” in REACH-IT
- Select “Create new” under the “Joint submission – registration” section
- At the “Contact details” step, click “Assign” next to the “Third party representative” field
- You can either:
- search for the TPR’s identity using the party’s name or UUID or
- select a TPR from the list of your previous TPRs in REACH-IT, after selecting, click “Assign”
- If you select “Yes” for the “Contact details publication acceptance”, then you give permission to ECHA to provide the identity of the assigned TPR (or the registrant if TPR is not appointed) to interested parties upon request. If you select “No”, then neither your, nor the TPR’s identity will be provided.
When submitting your registration dossier in REACH-IT:
- Click on “Menu” in REACH-IT
- Select “registration” as the type of dossier upload
- Upload the IUCLID dossier in the dossier submission wizard
- Continue to “Additional details”
- Click “Assign” next to “contact person”
- Click “Assign” next to the “Third party representative” field where you can assign an existing TPR or create a new one
- Continue to the “Confirmation” step of the wizard
Tasks for member registrants
As a member registrant if you don’t want your company name to be visible to your co-registrants, you must set a TPR when joining a joint submission and before submitting your registration dossier to ECHA.
Follow the below steps to assign a TPR when joining a joint submission:
- Click on “Menu” in REACH-IT
- Select “Join existing” joint submission
- Enter the joint submission name and security token
- Proceed to the ‘Contact details’ page. Here you have the option to create or assign an existing TPR
- Confirm your membership.
All the members of the joint submission will receive a notification in REACH-IT to inform them that a new member has joined the joint submission.
If a TPR is appointed, the members will see the name of the TPR in the “Members of the joint submission” field of the joint submission page.
You need to assign the TPR once more when you submit your registration dossier in REACH-IT:
- Click on “Menu” in REACH-IT
- Select “registration” as the type of dossier upload
- Upload the IUCLID dossier in the dossier submission wizard
- Continue to “Additional details”
- Click “Assign” next to “contact person”
- Click “Assign” next to the “Third party representative” field where you can assign an existing TPR or create a new one
- Continue to the “Confirmation” step of the wizard
- In the Co-registrants page,
- under the Potential registrants tab, if you included a TPR in a successful inquiry
- under the Registrants tab, if you included a TPR in a successful registration dossier
- In the Joint submission page
- if you included a TPR during creation (lead registrant) or
- if you included a TPR during confirmation (member registrant)
- In the pre-SIEF, if included in a pre-registration.
|
Claim type |
Substance is classified in accordance with REACH Art. 119(1)(a) |
Phase in status |
Dossier type |
Published if claimed confidential |
Fee required |
|
IUPAC-f |
✓ |
non-phase-in |
standard template, no INT/ sR&D or PPORD use |
✗ |
✓ |
|
IUPAC-g |
✓ |
not relevant |
INT template or standard template with only INT/ sR&D or PPORD use |
✗ |
✓ |
|
Non-hazardous IUPAC claim |
✗ |
not relevant |
not relevant |
✗ |
✗ |
|
Inadmissible IUPAC claim |
✓ |
phase-in |
standard template, no intermediate/ sR&D or PPORD use |
✓ |
✗ |
- Your claim is identified as an f-claim if you registered a non-phase-in substance which is classified in one of the hazard classes referred to in Article 119(1)(a). Your IUPAC name claim on such substances will be kept confidential for a period of 6 years.
- Your claim is identified as a g-claim if you registered a substance which is classified in one of the hazard classes referred to in Article 119(1)(a) and is used ONLY as an intermediate (INT), in scientific research and development (R&D), in product and process orientated research and development (PPORD). Your IUPAC name claim on such substances will be kept confidential for an indefinite period.
- Uses at Industrial sites (S3.5.3)
- Select the relevant status from the 'Registration/Notification status' picklist:
- use as intermediate registered according to REACH Article 10; total tonnage manufactured/imported >=10 tonnes/year per registrant or;
- use as intermediate registered according to REACH Article 10; total tonnage manufactured/imported <10 tonnes/year per registrant or;
- use registered according to REACH Article 17/18.
- If relevant, select the checkbox 'use In Scientific Research and Development' within 'Regulatory Status' field
- Uses by Professional workers (S3.5.4)
- If relevant, select the checkbox 'use in Scientific Research and Development' within 'Regulatory Status' field
- Consumer Uses (3.5.5)
- If relevant, select the checkbox 'use in Scientific Research and Development' within 'Regulatory Status' field
- In IUCLID’s web interface, set the flag next to the field ‘Legal entity’ or
- In IUCLID’s classic interface, set the flag above the field ‘Legal entity’.
If you would like to update the assigned Third Party Representative (TPR) in REACH-IT, you need to make changes in two places, on the reference number and the joint submission pages. Follow the below steps to update the TPR.
To update the TPR displayed on the reference number page, follow the steps below, this change will be visible also on the co-registrants page:
- Click on “Substances” in REACH-IT
- Search for the specific registration and click on the reference number to access the reference number page
- Here update your TPR under the “Third party representative” label within the contact information section
To update the TPR displayed in the joint submission page:
- If you are a member of a joint submission, click “View Joint Submission"
- Update your TPR in the “Joint submission contact” box under the title “Third party representative”

You can claim confidentiality on the registration number by ticking the box ‘Confidentiality claim on registration number’ in the IUCLID dossier header.

Alternatively, you can fill in a claim in section 1.3. Identifiers.

- the type of information and study results from the target and source records are always published. The IUCLID fields referring to results contain information e.g. indication of endpoint addressed, year and report date, test guideline, test results, remarks on results, etc.
- in the study document record, only the information contained in the field 'Specific details on test material used for the study' is not published when you claim a study record confidential
- inside the Test Material entity, only the information contained in the field ‘Details on test material’ is not published when you claim a study record confidential
- ECHA publishes the study result type (read-across, experimental result, etc.), as part of the results of the study, in accordance with article 119(1)(d) and (e) of REACH. However, the substance you read-across from, listed in the test material section, will not be published if the study summary is claimed confidential.
From the study document
|
Contents of the field |
Dissemination rule |
|
'Test material information' |
the link itself is published to enable viewing of the ‘Test material form’ information inside the TM entity
|
|
'Specific details on test material used for the study' |
not published if study record is flagged |
|
Specific details on test material used for the study (confidential) |
not published |
From the Test Material entity
|
Contents of the field |
Dissemination rule |
|
Test material name |
not published |
|
Type |
published |
|
Concentration and remarks |
not published |
|
Composition/purity |
not published |
|
Test material form |
published |
|
Details on test material |
not published if study record is flagged |
|
Confidential details on test material |
not published |
- legal entity;
- registration number;
- life cycle description and uses advised against;
- characterisation parameters of nanoforms;
- result of the PBT (Persistent, Bioaccumulative and Toxic chemicals) and vPvB (very Persistent and very Bioaccumulative) assessment;
- indication of whether a chemical safety assessment (CSA) was performed;
- article service life and article service life advised against;
- exposure scenario elements.
Safety data sheet information is published from all registration dossiers, whether the substance requires a safety data sheet or not, unless claimed confidential. Confidentiality needs to be claimed separately for each item, using the confidentiality flags in IUCLID. For substances, which do not require a safety data sheet, it is considered that the registrant ‘volunteers’ the safety data sheet information entered into IUCLID for publication, if the information is not claimed confidential.
Further information is available in Q&A 0412.
We currently do not publish the CSR itself.
A confidentiality claim in section 13, where the CSR is attached does not cover the CSR itself. This claim covers the fact whether a Chemical Safety Assessment was performed on the substance, as this information is present in the safety data sheet. Information that is part of the safety data sheet can be claimed as confidential in accordance with REACH Article 119(2)(d) Other information in the Safety Data Sheet.
Confidentiality claims on safety data sheet (SDS) information are only invoiced if the substance requires a safety data sheet. The fee for confidentiality claims on safety data sheet information is detailed in Annex IV of the Fee regulation.
If the substance requires a safety data sheet
The fee is charged only once per registration, regardless of the number of confidentiality claims on some or all of the specific items of SDS information. If the fee has been charged once, subsequently placed confidentiality claims on safety data sheet information will not be charged anymore. However, a specific justification for each of the types of information claimed confidential is still required.
It should be noted that confidentiality claims on the PBT assessment (section 2.3), on exposure scenarios and local assessment (section 3.5), and on whether a Chemical Safety Assessment (CSA) was performed (section 13) are invoiced if the substance requires a safety data sheet and the registrant submits a CSR.
Example:
If in a joint submission the lead registrant provides the Chemical Safety Report (CSR) on behalf of the members, only the lead will be invoiced for the before mentioned specific sections (i.e. provided that in the member dossier it is indicated that the CSR is submitted by the lead on behalf of the member). If a member individually submits the CSR, they will be charged for all potential SDS confidentiality claims (including sections 2.3, 3.5 and 13) as described above.
If the substance does not require a safety data sheet
It is deemed that the confidentiality claim indicates that the registrant does not volunteer the publication of the information, and no fee is charged for keeping this information confidential. Registrants can verify whether they will be charged for a confidentiality claim on safety data sheet information using the Fee Calculator in IUCLID.
Confidentiality claim on safety data sheet information for monomers
In accordance with the REACH Regulation, polymers are exempted from registration. However, monomer substances have to be registered under certain circumstances. A safety data sheet is needed for the monomer substance if it meets the relevant hazard criteria and it is placed on the European market. A safety data sheet for monomer substances is not required in the following situations:
- When the monomer is only imported as part of a polymer (as such or in a mixture).
- When the monomer is synthesized into the polymer directly by the manufacturer.
Consequently, a fee does not apply in these cases even if the safety data sheet related information is claimed confidential. In all other cases, a safety data sheet would be required for the monomer, as it will be made available from the manufacturer to a third party, and a fee would apply if the confidentiality on the safety data sheet is claimed.
If you receive an invoice for a confidentiality claim on safety data sheet information for a monomer which is covered by the conditions described above, please contact us via our contact form to explain your situation. We will analyse your case and provide you with instructions on how to proceed. The same applies for other substance(s) in the form of monomeric units and chemically bound substance(s).
Confidentiality claims accepted under Directive 67/548/EEC remain valid under REACH, as far as the information has not become available in the public domain. ECHA will assess the confidentiality claim and will – if the information is found to be publicly available – request further information before rejecting the claim.
You do not need to pay for claims that were already made under Directive 67/548/EEC. For more information, see How to update your previously notified substance (NONS), point 8. Updating your confidentiality requests.
- Degree of purity and the identity of dangerous impurities or additives:
- the confidentiality claim is made on the legal entity composition and;
- you reported at least one impurity or additive and you selected the tickbox 'this impurity/additive is considered relevant for the classification and labelling of the substance'.
- Tonnage band: you registered a substance with full (standard) dossier template.
- Robust study summaries and study summaries: you claimed confidentiality on a (robust) study summary record
- Safety Data Sheet (SDS) information: there are three main criteria to consider, (i) the registration type, (ii) whether a SDS is required for the substance and (iii) whether a Chemical Safety Report (CSR) is required.
The List of Safety Data Sheet claims with their respective rules (below the table you find the detailed description of the three main criteria):
|
Safety Data Sheet claim type |
Not only OSII tonnage |
SDS required |
CSR required |
|
Legal entity |
✓ |
✓ |
|
|
Registration number |
✓ |
✓ |
|
|
Use / Life cycle description and Use advised against |
✓ |
✓ |
|
|
Characterisation parameters of nanoforms |
✓ |
✓ |
|
|
Result of the PBT and vPvB assessment |
✓ |
✓ |
✓ |
|
Indication of whether a CSA was performed |
✓ |
✓ |
✓ |
|
Article service life and article service life advised against |
✓ |
✓ |
✓ |
|
Exposure scenario endpoint study record |
✓ |
✓ |
✓ |
- The substance is hazardous as per own Classification & Labelling (C&L) in S2.1 GHS. (If you are a member of a Joint submission, we check the lead’s GHS section);
- In S2.3 PBT assessment, the Substance is PBT/vPvB. (If you are a member of a Joint submission and you have not recorded a PBT assessment endpoint summary, we check the lead’s PBT assessment);
- The substance is listed on the candidate list of substances of very high concern for Authorisation .
- a CSR is required, if your standard tonnage band is above 10 tonnes/year.
- Trade name: you selected 'trade name' as identifier in the 'Other identifiers' table.
- IUPAC name: you registered a substance that is classified in accordance with Article 119(1)(a) and is non-phase-in or used ONLY as an intermediate (INT), in scientific research and development (R&D) or in product and process orientated research and development (PPORD). For further information on how to encode a IUPAC name claim in IUCLID, see Q&A 1771.
The fee paid for the confidentiality claim is to cover our work assessing your claim. If you decide to withdraw the claim, we are not able to refund the fee.
REACH applies to chemical substances and therefore only information about chemicals are disseminated on the ECHA webpage. It does not contain information about chemical preparations, formed by mixing different chemical substances, nor about articles containing chemicals.
In other words, you can find information about methanol or butane, but not for example about a shampoo, cleaning products or pencils.
Under the Waste Framework Directive (EU 2018/851), ECHA makes available information that suppliers submit on articles that contain substances of very high concern.
Most data disseminated on the ECHA website is retrieved from dossiers submitted by the industry. The Substance Infocards and Brief Profiles are automatically generated from the continually increasing quantity of chemical information submitted to ECHA. The quality and correctness of the information remains entirely the responsibility of the data submitter. ECHA is working with our stakeholders to improve the overall data quality.
The information submitted by the industry may or may not have been verified by ECHA. Under the evaluation processes, 20% of submitted dossiers are to be checked.
The information on chemicals subject to ECHA’s regulatory processes has been verified by the relevant experts before publication; this includes the Candidate list of SVHCs, the Authorisation list, and the list of substances restricted under REACH.
The following identifiers will produce search results.
Substance name
IUPAC names, CAS names, SMILE and InChI notation, trade names etc will produce results if present in submitted information and present on substance infocards.
EC number
The European Community number (numbers starting with 2, 3, 4 or 5) is a unique seven-digit identifier that was assigned to substances for regulatory purposes within the European Union by the European Commission or ECHA. You can find more information on these here.
List number
A List number (numbers starting with 6, 7, 8 or 9) is a technical identifier for managing submissions within ECHA. It is an automatically allocated number assigned by REACH-IT and therefore this number has no legal relevance as such.
CAS registry number
A CAS Registry Number, also referred to as CASRN or CAS Number, is a unique numerical identifier assigned by the Chemical Abstracts Service (CAS), a division of the American Chemical Society. These numerical identifiers are present in the ECHA databases but are not assigned by or in the remit of ECHA.
Index number
Index numbers relating to harmonised classifications present in Annex VI of the CLP legislation. These can relate to specific substances or group entries covering an array of substances fitting a group definition.
HS CODE
Harmonised System Code (e.g. 2903.89) used worldwide for customs and shipping
CN CODE
Combined Nomenclature (CN) (e.g. 2852 00 00) is the EU's eight-digit coding system, comprising the HS codes with further EU subdivisions. It both serves the EU's common customs tariff and provides statistics for trade inside the EU and between the EU and the rest of the world.
HS CODE MIXTURE
This is the HS code for a given substance when it’s a component of a mixture.
You should create an account on our website by clicking on ‘Sign in’ at the top of the page. Search for your substance using the ‘Search for Chemicals’ functionality. Once you have identified your substance of interest, click on the ‘star’ icon on the Infocard. This will allow you to receive weekly email alerts whenever information on your substance has changed.

- Addition of data from a new registrant (usually the identity of the registrant, and their specific compositions and uses for the substance).
- Update of information from an existing registrant:
- In case of an update by a member registrant, there may be changes to the specific composition or use.
- In case of an update by the lead registrant, additional scientific information may be included.
At times, individual members of substance joint submissions may have incorrectly indicated a substance as being a PBT substance. This then, by default, includes the substance in the PBT search results, but as a “minority position” i.e a minority of data submitters believe the substance to be PBT. As with all data present in registration dossiers, the responsibility for the accuracy of the data lies with the data submitters.
The advanced search option for PBT substances has several filter options available, which included Recognized and Majority position options in regard to PBT classification and employing such options will eliminate any substance incorrectly identified as PBT.

The ‘Advanced Search’ option has several options available to allow you to search for properties of concern.
These options can be combined with any of the other search functions of the Advanced Search, including most obviously, the substance identifiers section.

Substances may be grouped together under a specific regulatory activity for more efficient risk management and legislative processing (e.g. the same restriction on several asbestos fibres, or risk management analyses performed together for all isomers of a substance). Each group is defined by different criteria, fitting different regulatory purposes and/or risk management measures.
- Parent of a group
If a substance is itself the ‘parent’ of a group, an icon will be shown which provides a link to the list of identified members of that group.

- Member of a group
Group parents can potentially contain many individual substances as ‘group members’ or ‘children’ or other groups of substances.
If a substance is the member (or ‘child’) of at least one group, an icon will be shown to provide a link to the parent group substance to which the selected substance belongs.

Note that the list of groups or members to which a substance belongs will not be exhaustive, but will represent the groups which have been legally defined as containing the substance at the time the database was last updated.
Infocards are generated automatically based on the data available at the time of generation. If the links to other pages on a Substance Infocard (such as to the Brief profile or REACH registered substance factsheet) are displayed as grey, it means that no relevant information on that substance has not been submitted to ECHA. In the case of the link to REACH registered substance factsheets, this will mean there is no registration for that substance.

ECHA uses an algorithm to derive the substance master name, which is displayed in the search results and in the Infocard title. Since the available names are meaningless they are excluded. Therefore, the generic name "No public or meaningful name available" is assigned to be the primary name for the substance.
If and when any meaningful name becomes publicly available this will be taken into account by our algorithms and the substance master name will be updated.
The icons appearing in the Infocard in the “Hazard classification & labelling" section are calculated based on the number of notifications received from companies. For readability purposes, only the pictograms referred in more than 5% of the notifications under CLP are displayed. The full list of notified classifications is available in the C&L Inventory and in the ‘Hazard classification and labelling’ of the substance Brief Profile.
The table below defines which legal entity will be disseminated on ECHA’s webpage depending on information provided in sections 1.1 and 1.7 of your IUCLID dossier.


Further information can be found in the manual Dissemination and confidentiality under the REACH Regulation.
- where complete registration dossiers were submitted to ECHA by the registrant according to Article 20 of REACH;
- which were notified under Directive 67/548/EEC and the registration number was claimed by the notifier according to Article 24 of REACH.
The IUCLID Dissemination preview plug-in simulates which information from a registration dossier will be made public on the ECHA website, according to Article 119 of the REACH Regulation.
To use the tool, click on the button on the upper corner of your dossier → Dissemination preview.

- A tonnage band range, i.e., ≥100 000 – <1 000 000 tonnes, if no dossier contains a confidentiality claim on tonnage band;
- Open tonnage band, i.e., ≥1 000 tonnes, when at least one dossier contains a confidentiality claim on tonnage band;
- "Tonnage data confidential", when all dossiers aggregated have claimed their tonnage band as confidential, REACH Articles 119(2)(b); and
- "Intermediate use only", when only intermediate registration dossiers are present in the joint submission.
As you are responsible for using registered substances only, it is your interest to contractually ensure that your supplier guarantees the validity of their registration. Your first option is thus to ask directly your supplier.
If the supplier has not claimed the information as confidential you can search via the registrant name or the registration number from the Registered substances page.

Brief Profile
The full list of public active and inactive registrants/suppliers can be found in the Brief profile.

Fact sheet
The full list of public active and inactive registrants/suppliers/registration numbers can be found in the Fact sheet.

No. ECHA will not disseminate the manufacturing process description reported by registrants in compositions of the type ’legal entity composition of the substance’ in IUCLID section 1.2. This concerns both the information reported in the field ‘Description’ and any document included as ‘Attached description’.
Lead registrants need to additionally provide generic information on the manufacturing process of the jointly registered UVCB substance in the section 1.2 field ‘Description’ in compositions of the type ‘boundary composition of the substance’. This information will be displayed on the REACH-IT Joint submission page for the members of that joint submission to see.
The first place to check is the Registered Substances portal using a valid substance identifier.
A second option is to search via the Advanced Search function from our homepage.
Once you have found the substance you are looking for, you can see the names of registrants by viewing the information on that substance’s REACH Registered Substance Factsheet. The company names appear under “registrants/suppliers” of the Administrative Information section. In some cases, the list may not include your specific supplier, because:
- your supplier is not the one manufacturing or importing the substance; or
- the registrant has successfully claimed the name as confidential or
- the registrant is an importer covered by a representative of the non-EU exporter.
You can identify the REACH registration status of a registrant/supplier and of their registration numbers in the Administrative information section of the REACH factsheet.

There are four different statuses on the dissemination page:
- Active: The registration is valid, and the registrant is legally on the market.
- Cease manufacture: Inactive registrations after Cease of Manufacture.
- Annulled but still legally valid: In the case of a merge between two companies having a registration for the same substance, registration containing the higher tonnage band will be maintained as active, whereas the other registration as annulled. The annulled registration is still legally valid and considered as an active registration but is no longer updated.
- No longer valid: this can be due to the UK withdrawal from the EU, an incorrect declaration of company size, a retrospective completeness check failure or an invalid registration, when a Cease of Manufacture was communicated after the reception of a draft evaluation decision.
At the moment, it is only possible to download the whole registered substances database in batches. We suggest you first export the search results for all ‘individual’ submissions.

Once you have downloaded the relevant file, select ‘joint’ submissions as submission type.

Use the first published dates to limit the number of entries in a batch. Export the search results for all the registered substances published from 01/11/2008 to 30/11/2016. Export the second batch of registered substances published from 01/12/2016 to today’s date.

The Joint Submission is disseminated as intermediate only when all member registrants of the joint submission own an intermediate registration.
If any member registrant updates the registration from intermediate to full, the whole joint submission will be disseminated as full.
When extracting information from a submitted dossier, the dissemination process gives priority to data coming from dossiers registered as full. For this reason, the registration type displayed on the ECHA website is Full and the tonnage band is calculated using only data extracted from full type registrations as explained in Q&A 403.
You can see if members of a JS opted out of an endpoint by changing the view on the registered substance factsheet.

Although the lead registrant has changed for this substance, the data/test reports submitted by the previous lead registrant are not removed from our website. For transparency reasons, we publish all non-confidential data submitted by industry for a substance.
If at any time you would like any data submitted by the previous lead registrant to be removed from our website, we suggest you to contact the previous lead registrant and negotiate a registration update in order to remove specific data points.
ECHA does not reveal the identity of the Lead Registrants. You can find the contact details of the Lead Registrant in the co-registrants page in REACH-IT which you can access for a period of one year after a positive assessment of your inquiry.
For mor information see Q&A0457.
The revised Waste Framework Directive 2008/98/EC, which entered into force in July 2018, provided a role for ECHA to establish and maintain a database with information on substances of concern in articles, as such or in complex objects (products), named the “SCIP database”.
The SCIP database contains information on articles submitted by any EU supplier of an article with a duty to submit data according to Article 9(1)(i) of the Waste Framework Directive and Article 33(1) of the REACH Regulation.
The SCIP database provides information to identify articles containing one or more candidate list substance(s) of very high concern (SVHC) above 0.1 % (weight by weight), the location of SVHC substance(s) within an article and other information to allow the safe handling of an article, when necessary (see Q&A 1612).
The information submitted to the SCIP database is publicly available and therefore readily available to waste operators to bridge the current gap in the information flow.
The search results provide information on the latest information available from individual notifications submitted to the SCIP database. The factsheet of a notification is a snapshot in structured format of the information provided by notifiers to the SCIP database. A factsheet includes the following sections:
- An orange section (A) to provide an overview of key elements of a SCIP notification – the identifiers provided, a link to the safe use instructions, and a summary of the candidate list substances contained in the article;
- A tree structure (B) to describe the hierarchy of a complex object (article) and its components; and
- A central section (C) with identifiers, categorisation, safe use instructions and optional sections such as characteristics and number of units.
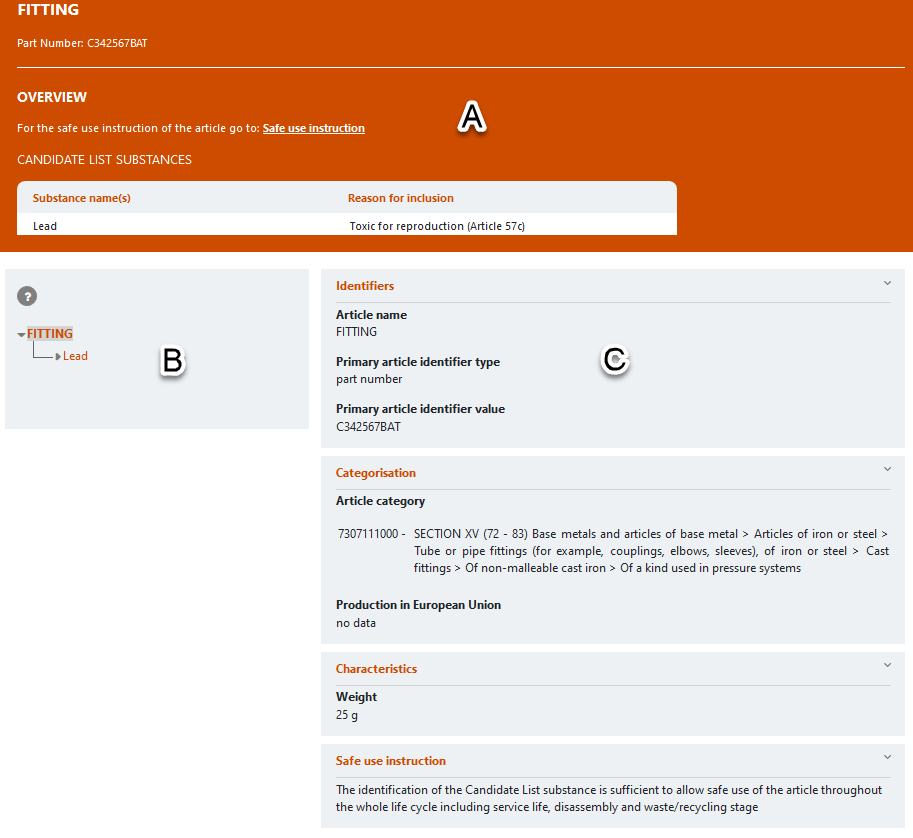
For complex object articles, components will be linked to form a hierarchy menu: all subcomponents within a hierarchy will need to be opened to identify the SVHC substance at the end of the hierarchy.

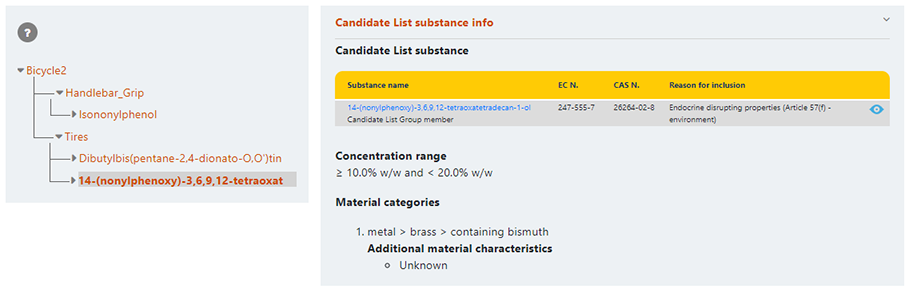
Additional information will be displayed when clicking on a component or candidate list substance header.
Notifications to the SCIP database follow a validation process that ensures that the format of SCIP notifications is correct, however the correctness of the information provided remains the sole responsibility of the data submitters.
The information published in the SCIP database is not reviewed or verified by ECHA and can be changed by the data submitter at any time without prior notice. ECHA does not guarantee the correctness of the information published in the database as it is automatically published based on notifications submitted.
A set of Q&As on general SCIP subjects can be found at: Waste Framework Directive - SCIP database
The SCIP database provides information on articles based on notifications submitted by notifiers to the SCIP database. Notifiers have two options:
- One notification corresponds to one article
- One notification corresponds to several articles. Different variations of the same article can be included in the same notification provided that the notifiers fulfil the criteria for grouping them in the same notification.
When several articles are part of one notification, it is expected that the combination of article identifier and characteristics are available to allow users of the SCIP database discern the specific article they are looking for. It is equally important that commercially available identifiers are included in a notification, whenever applicable and provided by the relevant notifier.
Additional useful information and the criteria defined for grouping can also be found in Section 3 of the guide ‘Requirements for SCIP notifications’ and Q&A 1845.
It is recommended that you follow these principles when searching in the SCIP database:
- Use commercially available standard identifiers as available in labels or catalogues, such a standard identifier, brand, or model. Be prepared to try a number of identifiers in case the one you have selected has not been included in any notification.
- When too many results are displayed, consider refining your search criteria, e.g., select a specific article category or substance of very high concern (SVHC), when already known to you.
Additional search criteria can be considered such as material & mixture category, substance of concern or reasons for inclusion (reasons for an SVHC having been added to the Candidate List (e.g., carcinogenic.)
The adequacy of the information provided to the SCIP database according to ECHA’s guidance on submission of SCIP notifications remains the responsibility of notifiers to the SCIP database.
The obligation to submit notifications to the SCIP database is applicable to all suppliers of an article within the supply chain, independently of whether an article is distributed without any further change or an article is considered as part of the assembling process. In the event that the same article is distributed within the supply chain, duplicates may be expected. Different reasons may apply, namely:
- Notifiers do not make use of the voluntary tools to refer to data already submitted e.g., SSN tool (see Q&A 1843).
- Notifiers submit their own SCIP notification before other actors in the supply chain make their notifications, so the tools to refer to data already submitted cannot be used (e.g., a supplier has not notified yet to SCIP)
- Notifiers prefer using their own standard primary article identifier to make their own notification visible in the SCIP database.
Other reasons may apply. The content of a duplicate article is expected to be the same. You may find a different (standard) primary article identifier or other information changed, depending on the reasons why a notifier decides to submit their own SCIP notification. You can consider narrowing down the search criteria using the standard primary article identifier, when available. If exact duplicates are available, it is not relevant which one is correct. In certain scenarios, different results may display the same article information but different parts numbers: this is an indication that distributors may have decided to use their own identifiers, but the article remains the same.
SCIP numbers are alphanumerical identifiers used by notifiers to the SCIP database to identify unambiguously unique SCIP notifications available in the SCIP database. Searches can now be carried out by duty holders based on exact SCIP numbers.
Please note that SCIP number searches should be done using the appropriate search filter and not the option under Article identity.

If you are searching for a SCIP number based on simplified notification (SSN) tools, it is important to consider that:
- Searches in the SCIP database cannot be made based on a notifier’s name.
- Only exact SCIP numbers can be searched for.
- If the number provided to you has been provided in error, you need to contact your supplier.
- A submission based on SSN provides notifiers the possibility to simply make reference to an already existing notification submitted to the SCIP database. Since no new content is provided in a SSN notification and the content is already available in the reference application, ECHA will publish only the content available in the reference notification.
The information required for the SCIP database must already be communicated throughout the supply chain under REACH Article 33(1). Consequently, the SCIP database complements the existing communication obligations to be considered between suppliers and downstream users. (See Q&A 1618)
Furthermore, the information published in the SCIP database is subject to confidentiality rules as described in the manual Dissemination and confidentiality in the SCIP database. In the event that additional information was needed in SCIP format, it will be the up to the supplier to provide their downstream users with the SCIP notifications (or produce IUCLID reports with or without sensitive information).
Because there are hundreds or thousands of options in the pick lists for article, material and mixture category, and likewise for substances of concern, there is a new ‘pre-search’ functionality.
In the pre-search field enter a text string of interest (a). It is important to click on the pre-search ‘search’ button (b); pressing Enter will trigger the main search for articles prematurely.
After searching for your text string of interest any matching results will be returned, highlighted in yellow as shown below (d). It is necessary that you select one or more pick-values, scrolling through the list of results (c), as necessary.
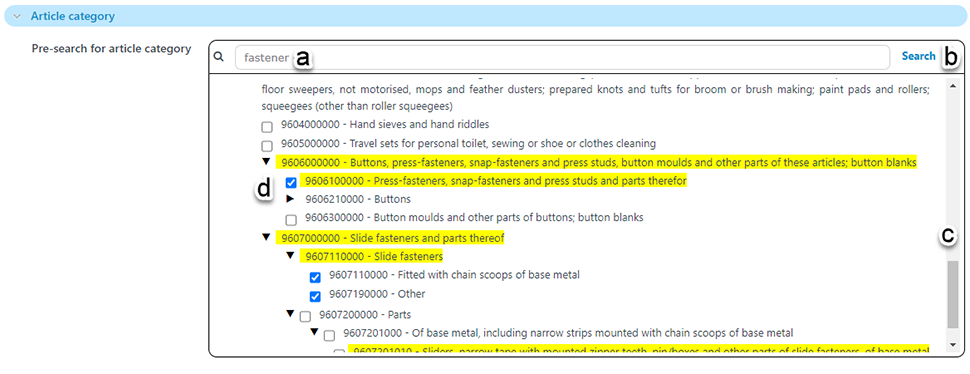
Once you have selected one or more values of interest, trigger the main search for articles to find any matching your selected criteria.

Remember to clear all previous search criteria before attempting a fresh search.
ECHA will make automatic daily updates to the information shown in the SCIP database based on data submissions. In order to successfully process the large volumes of data received some time is necessary – for validation, checking, preparing search indexes, publishing and so on. For this reason, be aware that a given submission is unlikely to be available on the public website until after a few days have passed.
The work carried out by ECHA to publish information on the SCIP database involves, among other things, the processing of large volumes of data, handling of data among different systems, synchronisation of available data based on the latest update(s) available and referential checks.
The message “data pending” in a complex object component refers to a link established by a SCIP notifier to pieces of information available in other SCIP notifications. As soon as the target SCIP notification is processed and available, the “Data pending” message will be automatically updated with the actual data that is referenced.


