Q&As
Q&As
Q&A info
Möchten Sie die jeweilige Frage und Antwort in Ihrer Sprache suchen? Ändern Sie die Sprache in der Auswahlliste oben.
Zurück
- Validation assistant, which enables you to validate your registration dossier before submitting it, i.e. it performs a Business Rule check to ensure that you included a justification along your confidentiality claim;
- Dissemination Preview tool, which simulates which information from your dossier will be published on the ECHA website;
- Fee Calculator tool, which calculates the costs of your confidentiality claims and any other fees associated to your registration.
Indeed, we send the decision (acceptance/rejection) of a confidentiality claim via REACH-IT at the end of the assessment procedure.
If the justification is not sufficient, you will receive a communication detailing which information needs to be updated in order for your claim to be accepted. During the assessment of the confidentiality claim, the information claimed confidential is not published on the ECHA website.
After the assessment is concluded, the information found to be validly claimed confidential will not be made public. However, in case of rejected confidentiality claims, the information will be published on ECHA’s website.
For further information on the assessment procedure, see the Dissemination and Confidentiality under the REACH Regulation manual, section 3.8.
Please see our explanatory document on How to protect you confidential business information, section 1.
ECHA combines data per substance from all regulatory activities, therefore the information may be made publicly available on the ECHA website if not claimed to be kept confidential in all submissions. I.e. if another company submits e.g. a C&L notification or a registration for the same substance without claiming confidentiality on the substance name, it is published on the ECHA website.
Also, the validity of a confidentiality claim may be limited in time. If the validity of a confidentiality claim has expired in any of the submissions, the corresponding information is published on the ECHA website.
If you are not sure why the information is published, you can contact us.
Each item falling under REACH Article 119(2) must be separately claimed in the dossier and the reason for confidentiality must be justified also separately for each of them. The validity of these confidentiality claims is assessed.
The justification for each confidentiality claim needs to include: (1) a declaration that the information is not in the public domain or general knowledge in the industry, (2) a demonstration of a commercial interest worthy of protection for non-disclosure of the information, and (3) a demonstration that disclosure of the information would cause potential harm to the commercial interest of the registrant or a third party.
To claim confidentiality and insert the justification template in IUCLID web interface, open the flag next to the item you wish to keep confidential and select the confidentiality flag; CBI, IP or no PA. The template for justifying a confidentiality claim can be directly inserted in the justification field by selecting ‘Insert existing templates’ on the top right corner of the justification text box. After inserting the template, you can start editing it.
For dossier updates, justifications previously attached to the dossier in pdf format can be maintained.
- Ensure that the information claimed confidential is not already publicly available elsewhere.
- Add the CBI flag next to the information you wish to claim confidential and provide a valid justification.
- Run the Dissemination preview tool both in your IUCLID dataset and created dossier, to see which information from your registration dossier will be removed before publication and which information will be publicly available.
- Run the Fee calculator to see the applicable fees you need to pay.
Please contact us via our contact form, as soon as you notice that the information has not been flagged as confidential.
In the meantime, your latest successfully submitted dossier will be published and only the flagged confidential information will be removed from publication, pending confidentiality assessment.
You will be informed of the confidentiality assessment outcome via REACH-IT. In case of acceptance, the information you wish to not make publicly available will remain confidential.
In case of rejection, you will be asked further information before the confidentiality assessment is finalised and before the information is published.
Under REACH, only information entered into a IUCLID field is published. Attachments are not published.
- Click on “Menu” in REACH-IT
- Select “inquiry” as the type of dossier upload
- Upload the IUCLID dossier in the dossier submission wizard
- Continue to “Additional details”
- Click “Assign” next to “contact person”
- Click “Assign” next to the “Third party representative” field where you can assign an existing TPR or create a new one
- Continue to the “Confirmation” step of the wizard
Tasks for lead registrants
As a lead registrant, you can assign a TPR for the joint submission before submitting your registration dossier. Follow the below steps to assign a TPR for the joint submission:
- Click on “Menu” in REACH-IT
- Select “Create new” under the “Joint submission – registration” section
- At the “Contact details” step, click “Assign” next to the “Third party representative” field
- You can either:
- search for the TPR’s identity using the party’s name or UUID or
- select a TPR from the list of your previous TPRs in REACH-IT, after selecting, click “Assign”
- If you select “Yes” for the “Contact details publication acceptance”, then you give permission to ECHA to provide the identity of the assigned TPR (or the registrant if TPR is not appointed) to interested parties upon request. If you select “No”, then neither your, nor the TPR’s identity will be provided.
When submitting your registration dossier in REACH-IT:
- Click on “Menu” in REACH-IT
- Select “registration” as the type of dossier upload
- Upload the IUCLID dossier in the dossier submission wizard
- Continue to “Additional details”
- Click “Assign” next to “contact person”
- Click “Assign” next to the “Third party representative” field where you can assign an existing TPR or create a new one
- Continue to the “Confirmation” step of the wizard
Tasks for member registrants
As a member registrant if you don’t want your company name to be visible to your co-registrants, you must set a TPR when joining a joint submission and before submitting your registration dossier to ECHA.
Follow the below steps to assign a TPR when joining a joint submission:
- Click on “Menu” in REACH-IT
- Select “Join existing” joint submission
- Enter the joint submission name and security token
- Proceed to the ‘Contact details’ page. Here you have the option to create or assign an existing TPR
- Confirm your membership.
All the members of the joint submission will receive a notification in REACH-IT to inform them that a new member has joined the joint submission.
If a TPR is appointed, the members will see the name of the TPR in the “Members of the joint submission” field of the joint submission page.
You need to assign the TPR once more when you submit your registration dossier in REACH-IT:
- Click on “Menu” in REACH-IT
- Select “registration” as the type of dossier upload
- Upload the IUCLID dossier in the dossier submission wizard
- Continue to “Additional details”
- Click “Assign” next to “contact person”
- Click “Assign” next to the “Third party representative” field where you can assign an existing TPR or create a new one
- Continue to the “Confirmation” step of the wizard
- In the Co-registrants page,
- under the Potential registrants tab, if you included a TPR in a successful inquiry
- under the Registrants tab, if you included a TPR in a successful registration dossier
- In the Joint submission page
- if you included a TPR during creation (lead registrant) or
- if you included a TPR during confirmation (member registrant)
- In the pre-SIEF, if included in a pre-registration.
|
Claim type |
Substance is classified in accordance with REACH Art. 119(1)(a) |
Phase in status |
Dossier type |
Published if claimed confidential |
Fee required |
|
IUPAC-f |
✓ |
non-phase-in |
standard template, no INT/ sR&D or PPORD use |
✗ |
✓ |
|
IUPAC-g |
✓ |
not relevant |
INT template or standard template with only INT/ sR&D or PPORD use |
✗ |
✓ |
|
Non-hazardous IUPAC claim |
✗ |
not relevant |
not relevant |
✗ |
✗ |
|
Inadmissible IUPAC claim |
✓ |
phase-in |
standard template, no intermediate/ sR&D or PPORD use |
✓ |
✗ |
- Your claim is identified as an f-claim if you registered a non-phase-in substance which is classified in one of the hazard classes referred to in Article 119(1)(a). Your IUPAC name claim on such substances will be kept confidential for a period of 6 years.
- Your claim is identified as a g-claim if you registered a substance which is classified in one of the hazard classes referred to in Article 119(1)(a) and is used ONLY as an intermediate (INT), in scientific research and development (R&D), in product and process orientated research and development (PPORD). Your IUPAC name claim on such substances will be kept confidential for an indefinite period.
- Uses at Industrial sites (S3.5.3)
- Select the relevant status from the 'Registration/Notification status' picklist:
- use as intermediate registered according to REACH Article 10; total tonnage manufactured/imported >=10 tonnes/year per registrant or;
- use as intermediate registered according to REACH Article 10; total tonnage manufactured/imported <10 tonnes/year per registrant or;
- use registered according to REACH Article 17/18.
- If relevant, select the checkbox 'use In Scientific Research and Development' within 'Regulatory Status' field
- Uses by Professional workers (S3.5.4)
- If relevant, select the checkbox 'use in Scientific Research and Development' within 'Regulatory Status' field
- Consumer Uses (3.5.5)
- If relevant, select the checkbox 'use in Scientific Research and Development' within 'Regulatory Status' field
- In IUCLID’s web interface, set the flag next to the field ‘Legal entity’ or
- In IUCLID’s classic interface, set the flag above the field ‘Legal entity’.
If you would like to update the assigned Third Party Representative (TPR) in REACH-IT, you need to make changes in two places, on the reference number and the joint submission pages. Follow the below steps to update the TPR.
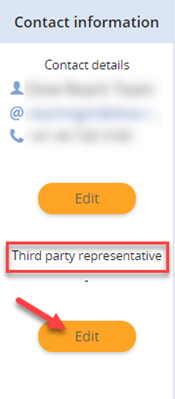
To update the TPR displayed on the reference number page, follow the steps below, this change will be visible also on the co-registrants page:
- Click on “Substances” in REACH-IT
- Search for the specific registration and click on the reference number to access the reference number page
- Here update your TPR under the “Third party representative” label within the contact information section
To update the TPR displayed in the joint submission page:
- If you are a member of a joint submission, click “View Joint Submission"
- Update your TPR in the “Joint submission contact” box under the title “Third party representative”

You can claim confidentiality on the registration number by ticking the box ‘Confidentiality claim on registration number’ in the IUCLID dossier header.

Alternatively, you can fill in a claim in section 1.3. Identifiers.

- the type of information and study results from the target and source records are always published. The IUCLID fields referring to results contain information e.g. indication of endpoint addressed, year and report date, test guideline, test results, remarks on results, etc.
- in the study document record, only the information contained in the field 'Specific details on test material used for the study' is not published when you claim a study record confidential
- inside the Test Material entity, only the information contained in the field ‘Details on test material’ is not published when you claim a study record confidential
- ECHA publishes the study result type (read-across, experimental result, etc.), as part of the results of the study, in accordance with article 119(1)(d) and (e) of REACH. However, the substance you read-across from, listed in the test material section, will not be published if the study summary is claimed confidential.
From the study document
|
Contents of the field |
Dissemination rule |
|
'Test material information' |
the link itself is published to enable viewing of the ‘Test material form’ information inside the TM entity
|
|
'Specific details on test material used for the study' |
not published if study record is flagged |
|
Specific details on test material used for the study (confidential) |
not published |
From the Test Material entity
|
Contents of the field |
Dissemination rule |
|
Test material name |
not published |
|
Type |
published |
|
Concentration and remarks |
not published |
|
Composition/purity |
not published |
|
Test material form |
published |
|
Details on test material |
not published if study record is flagged |
|
Confidential details on test material |
not published |
- legal entity;
- registration number;
- life cycle description and uses advised against;
- characterisation parameters of nanoforms;
- result of the PBT (Persistent, Bioaccumulative and Toxic chemicals) and vPvB (very Persistent and very Bioaccumulative) assessment;
- indication of whether a chemical safety assessment (CSA) was performed;
- article service life and article service life advised against;
- exposure scenario elements.
Safety data sheet information is published from all registration dossiers, whether the substance requires a safety data sheet or not, unless claimed confidential. Confidentiality needs to be claimed separately for each item, using the confidentiality flags in IUCLID. For substances, which do not require a safety data sheet, it is considered that the registrant ‘volunteers’ the safety data sheet information entered into IUCLID for publication, if the information is not claimed confidential.
Further information is available in Q&A 0412.
We currently do not publish the CSR itself.
A confidentiality claim in section 13, where the CSR is attached does not cover the CSR itself. This claim covers the fact whether a Chemical Safety Assessment was performed on the substance, as this information is present in the safety data sheet. Information that is part of the safety data sheet can be claimed as confidential in accordance with REACH Article 119(2)(d) Other information in the Safety Data Sheet.
Confidentiality claims on safety data sheet (SDS) information are only invoiced if the substance requires a safety data sheet. The fee for confidentiality claims on safety data sheet information is detailed in Annex IV of the Fee regulation.
If the substance requires a safety data sheet
The fee is charged only once per registration, regardless of the number of confidentiality claims on some or all of the specific items of SDS information. If the fee has been charged once, subsequently placed confidentiality claims on safety data sheet information will not be charged anymore. However, a specific justification for each of the types of information claimed confidential is still required.
It should be noted that confidentiality claims on the PBT assessment (section 2.3), on exposure scenarios and local assessment (section 3.5), and on whether a Chemical Safety Assessment (CSA) was performed (section 13) are invoiced if the substance requires a safety data sheet and the registrant submits a CSR.
Example:
If in a joint submission the lead registrant provides the Chemical Safety Report (CSR) on behalf of the members, only the lead will be invoiced for the before mentioned specific sections (i.e. provided that in the member dossier it is indicated that the CSR is submitted by the lead on behalf of the member). If a member individually submits the CSR, they will be charged for all potential SDS confidentiality claims (including sections 2.3, 3.5 and 13) as described above.
If the substance does not require a safety data sheet
It is deemed that the confidentiality claim indicates that the registrant does not volunteer the publication of the information, and no fee is charged for keeping this information confidential. Registrants can verify whether they will be charged for a confidentiality claim on safety data sheet information using the Fee Calculator in IUCLID.
Confidentiality claim on safety data sheet information for monomers
In accordance with the REACH Regulation, polymers are exempted from registration. However, monomer substances have to be registered under certain circumstances. A safety data sheet is needed for the monomer substance if it meets the relevant hazard criteria and it is placed on the European market. A safety data sheet for monomer substances is not required in the following situations:
- When the monomer is only imported as part of a polymer (as such or in a mixture).
- When the monomer is synthesized into the polymer directly by the manufacturer.
Consequently, a fee does not apply in these cases even if the safety data sheet related information is claimed confidential. In all other cases, a safety data sheet would be required for the monomer, as it will be made available from the manufacturer to a third party, and a fee would apply if the confidentiality on the safety data sheet is claimed.
If you receive an invoice for a confidentiality claim on safety data sheet information for a monomer which is covered by the conditions described above, please contact us via our contact form to explain your situation. We will analyse your case and provide you with instructions on how to proceed. The same applies for other substance(s) in the form of monomeric units and chemically bound substance(s).
Confidentiality claims accepted under Directive 67/548/EEC remain valid under REACH, as far as the information has not become available in the public domain. ECHA will assess the confidentiality claim and will – if the information is found to be publicly available – request further information before rejecting the claim.
You do not need to pay for claims that were already made under Directive 67/548/EEC. For more information, see How to update your previously notified substance (NONS), point 8. Updating your confidentiality requests.
- Degree of purity and the identity of dangerous impurities or additives:
- the confidentiality claim is made on the legal entity composition and;
- you reported at least one impurity or additive and you selected the tickbox 'this impurity/additive is considered relevant for the classification and labelling of the substance'.
- Tonnage band: you registered a substance with full (standard) dossier template.
- Robust study summaries and study summaries: you claimed confidentiality on a (robust) study summary record
- Safety Data Sheet (SDS) information: there are three main criteria to consider, (i) the registration type, (ii) whether a SDS is required for the substance and (iii) whether a Chemical Safety Report (CSR) is required.
The List of Safety Data Sheet claims with their respective rules (below the table you find the detailed description of the three main criteria):
|
Safety Data Sheet claim type |
Not only OSII tonnage |
SDS required |
CSR required |
|
Legal entity |
✓ |
✓ |
|
|
Registration number |
✓ |
✓ |
|
|
Use / Life cycle description and Use advised against |
✓ |
✓ |
|
|
Characterisation parameters of nanoforms |
✓ |
✓ |
|
|
Result of the PBT and vPvB assessment |
✓ |
✓ |
✓ |
|
Indication of whether a CSA was performed |
✓ |
✓ |
✓ |
|
Article service life and article service life advised against |
✓ |
✓ |
✓ |
|
Exposure scenario endpoint study record |
✓ |
✓ |
✓ |
- The substance is hazardous as per own Classification & Labelling (C&L) in S2.1 GHS. (If you are a member of a Joint submission, we check the lead’s GHS section);
- In S2.3 PBT assessment, the Substance is PBT/vPvB. (If you are a member of a Joint submission and you have not recorded a PBT assessment endpoint summary, we check the lead’s PBT assessment);
- The substance is listed on the candidate list of substances of very high concern for Authorisation .
- a CSR is required, if your standard tonnage band is above 10 tonnes/year.
- Trade name: you selected 'trade name' as identifier in the 'Other identifiers' table.
- IUPAC name: you registered a substance that is classified in accordance with Article 119(1)(a) and is non-phase-in or used ONLY as an intermediate (INT), in scientific research and development (R&D) or in product and process orientated research and development (PPORD). For further information on how to encode a IUPAC name claim in IUCLID, see Q&A 1771.
The fee paid for the confidentiality claim is to cover our work assessing your claim. If you decide to withdraw the claim, we are not able to refund the fee.


