All news
-
News
- Image gallery
- Video library
- Safer chemicals podcast
-
Hot topics
- Preventing cancer
- Skin sensitising chemicals
- Per- and polyfluoroalkyl substances (PFAS)
- Microplastics
- Granules and mulches on sports pitches and playgrounds
- Tattoo inks and permanent make-up
- Glyphosate
- Endocrine disruptors
- Bisphenols
- Chemicals Strategy for Sustainability
- Alternatives to animal testing
- Phthalates
- Biocides
- Lead
- Research to enhance protection of our health and environment
- Corporate and visual Identity
- ECHA Articles
All news
236 substances shortlisted for possible regulatory action
ECHA/NR/18/03
ECHA has selected 236 substances for further scrutiny by the Member State competent authorities in its annual screening exercise. The competent authorities will carry out a manual examination of dossiers they prioritise to decide whether regulatory action is needed.
Helsinki, 23 January 2018 – If your company has registered one of the substances now shortlisted, you will receive a letter from ECHA informing you of the potential examination of your registration. We encourage you to update your dossiers to address any shortcomings as soon as possible. Up-to-date information will help the Member State authorities better assess if regulatory action – either further data generation or regulatory risk management measures - is needed.
You are invited to participate in our webinar taking place on 1 February. In this webinar, you will get more details about the screening process, and the next steps. You will also have the opportunity to ask ECHA staff questions.
If the Member States or ECHA take actions on your substance, this information will be published on the Agency’s website, for example, in the list of substances potentially subject to compliance checks, the Registry of Intentions (RoI), the draft CoRAP for substance evaluation, or the Public Activities Coordination Tool (PACT). You can check the status of your substance through the Search for chemicals functionality available on ECHA’s homepage. ECHA does not make the list of shortlisted substances public as it is based on automated selection and manual verification is needed to confirm a potential concern.
How substances are selected for the shortlist
The selection is mostly based on an approach where groups are created around substances with suspected or known concerns, such as those on the Community rolling action plan (CoRAP) for substance evaluation or on the Candidate List of substances of very high concern (SVHCs). Substances are selected to the groups based on read-across arguments and categories available in registration dossiers or categories used in other regulatory programmes (e.g. OECD), as well as structural similarity.
Addressing substances in groups will make sure similar substances are treated consistently. The grouping extends beyond the REACH registered substances and the current shortlist includes 40 substances notified to the C&L Inventory, as well as the 39 group seeds (substances with suspected or know concerns) included on the CoRAP or Candidate List, and therefore already being looked at.
Background
ECHA and the Member State competent authorities annually conduct IT-based and manual screenings of registered substances as part of a common screening approach. The aim is to identify substances that pose a risk for human health or the environment, and take them forward to the most appropriate REACH and CLP processes to ensure their safe use. The common screening approach is part of ECHA’s integrated regulatory strategy.
The previous four rounds of IT screening have identified altogether 1 084 substances for further scrutiny. Of those, Member States have examined 714 substances and three-quarters of them required follow-up activities. See the graph below for more information.
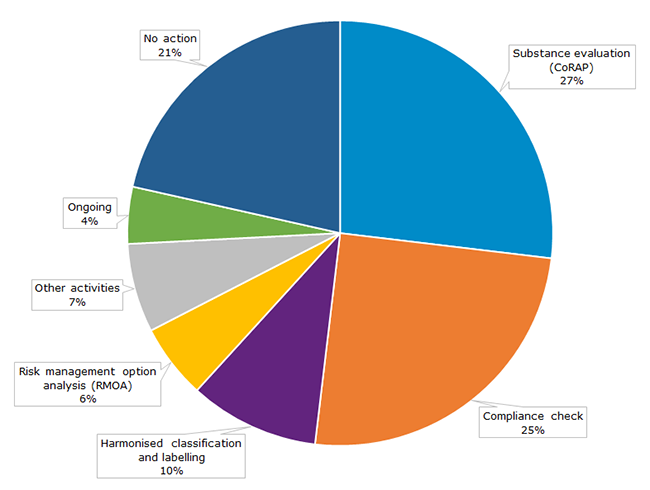
Graph: Outcomes of the four rounds of substance screenings (2014-2017).


